
How many single bonds can boron form?
Answer
557.1k+ views
Hint: We know that boron is an element whose atomic number is 5. It belongs to group 13 of the periodic table. It is chemically represented as B. Here, we have to find the number of single bonds formed by the boron.
Complete step by step answer:
Let’s first discuss the octet rule in detail. According to this rule, the atoms of different elements take part in chemical combination in order to complete octet (to have eight electrons in the outermost shell) or duplet (to have two valence electrons such as in case of H, Li etc.)
Let’s come to the case of B. The electronic configuration of boron is 2,3. Boron can share its 3 electrons with three fluorine atoms to form a stable compound. But it does not follow the octet rule.
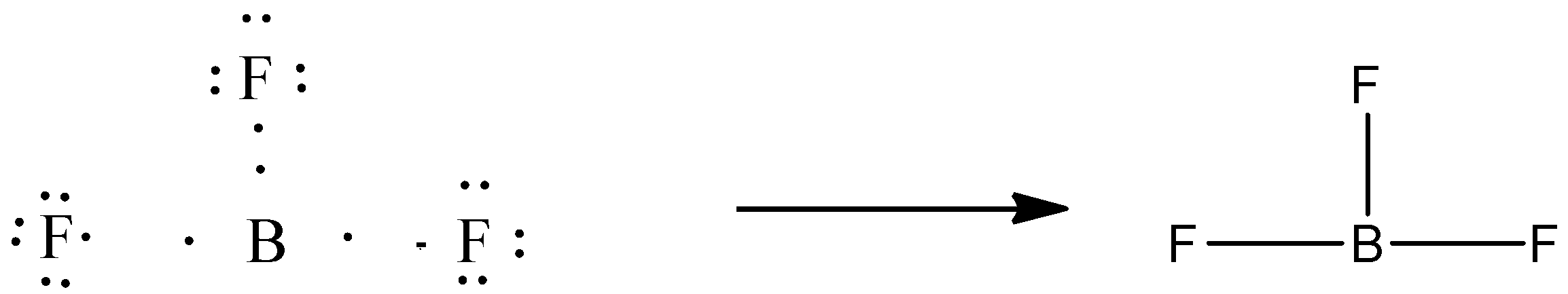
So, the number of single covalent bonds formed by boron is three.
Additional Information:
We know that there are two modes of chemical combination, that is, by electron transfer and by sharing electrons. The chemical bond formed by sharing electrons is termed as covalent bond and when electron transfer results in chemical bond, then the chemical bond is termed as ionic bond.
Note: It is to be noted that there are many limitations of octet rule, such as incomplete octet of central atoms such as in case of ${\text{B}}{{\text{F}}_{\text{3}}}$, ${\text{BeC}}{{\text{l}}_{\text{2}}}$ etc. Also in some molecules, there is expanded octet, such as in case of ${\text{P}}{{\text{F}}_{\text{5}}}$ where 10 electrons present around P atom. Also the octet rule does not give any idea about the shape of the molecules.
Complete step by step answer:
Let’s first discuss the octet rule in detail. According to this rule, the atoms of different elements take part in chemical combination in order to complete octet (to have eight electrons in the outermost shell) or duplet (to have two valence electrons such as in case of H, Li etc.)
Let’s come to the case of B. The electronic configuration of boron is 2,3. Boron can share its 3 electrons with three fluorine atoms to form a stable compound. But it does not follow the octet rule.
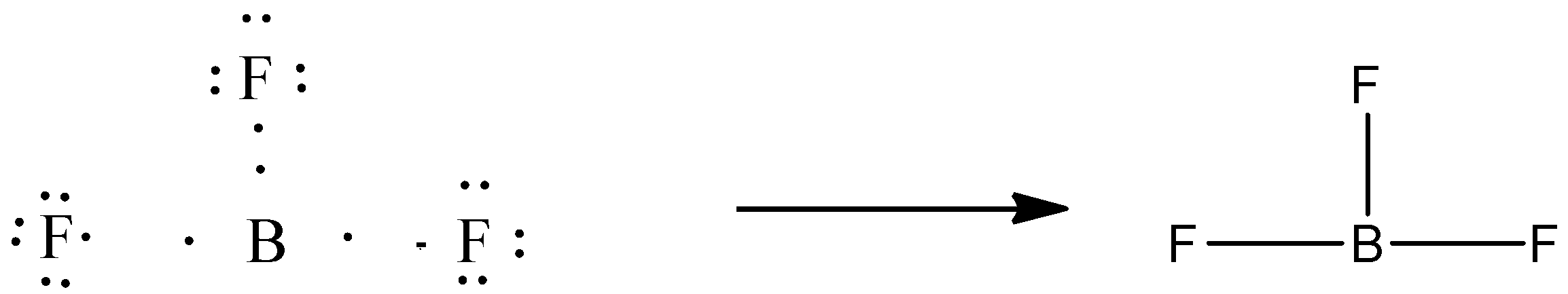
So, the number of single covalent bonds formed by boron is three.
Additional Information:
We know that there are two modes of chemical combination, that is, by electron transfer and by sharing electrons. The chemical bond formed by sharing electrons is termed as covalent bond and when electron transfer results in chemical bond, then the chemical bond is termed as ionic bond.
Note: It is to be noted that there are many limitations of octet rule, such as incomplete octet of central atoms such as in case of ${\text{B}}{{\text{F}}_{\text{3}}}$, ${\text{BeC}}{{\text{l}}_{\text{2}}}$ etc. Also in some molecules, there is expanded octet, such as in case of ${\text{P}}{{\text{F}}_{\text{5}}}$ where 10 electrons present around P atom. Also the octet rule does not give any idea about the shape of the molecules.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




