
Select the correct statement (s) about the compound ${{N}}{{{O}}^{{ + }}}{{{[B}}{{{F}}_{{4}}}{{]}}^{{ - }}}$
This question has multiple correct answers.
(A) It has ${{5\sigma\; and\; 2\pi }}$ bonds.
(B) Nitrogen –oxygen bond length is higher than the nitric oxide (NO) .
(C) It is a diamagnetic species.
(D) B – F bond length in this compound is lower than in ${{B}}{{{F}}_{{3}}}$.
Answer
558.3k+ views
Hint: Before moving towards the options, we have to draw the structure of the compound, i.e. ${{N}}{{{O}}^{{ + }}}{{{[B}}{{{F}}_{{4}}}{{]}}^{{ - }}}$. Then we will analyze the number of bonds, their bond order, electron pairing and the length of the bonds between them. Diamagnetism or paramagnetism depends upon the number of electrons which is unpaired.
Complete step by step answer:
First let us see the structure of the compound.
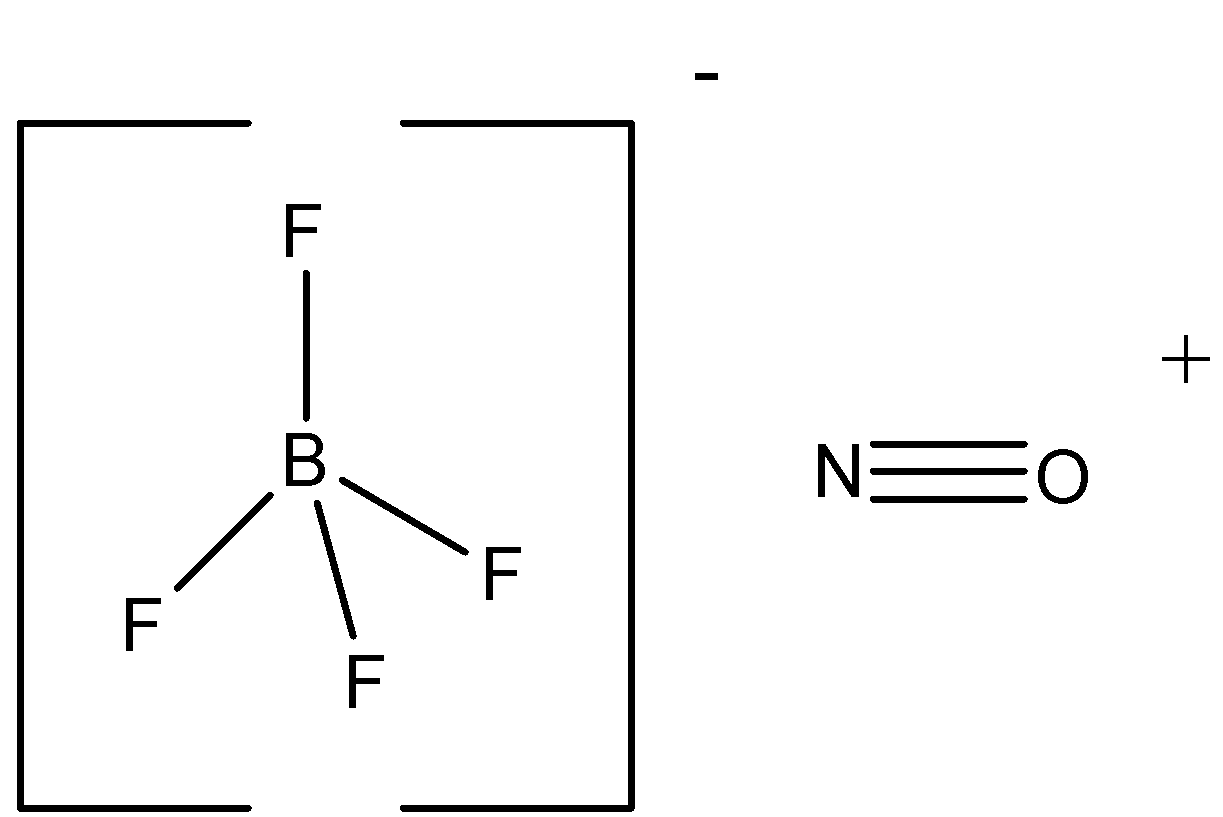
The structure shows us the number of bonds between the constituent atoms. Let’s see each options statement:
(A) According to the structure above the ${{{[B}}{{{F}}_{{4}}}{{]}}^{{ - }}}{{ has\; 4\sigma }}$ bonds and ${{N}}{{{O}}^{{ + }}}{{ has\; 1\sigma \; and \;2\pi }}$ bonds as a whole it has five sigma bonds and two pi bonds which is correct. So, option A is correct.
(B) The nitrosonium ion here has a bond order of three and nitric oxide has a bond order of ${{2}}{{.5}}$. We already know that more bonds mean higher bond order so more strength and short bond length. Since nitrosonium cation has more bond – order so less bond length than NO. So this option is incorrect.
(C) Since all the electrons in this structure are even and paired up so there is only diamagnetic nature in the compound. So, this option is correct.
(D) The ${{p\pi - p\pi }}$ back bonding occurs when the ligand is electron rich lone pairs in p – orbitals and metal in centre have vacant p – orbital then back bonding occur which is present in ${{B}}{{{F}}_{{3}}}{{ \;and\; not\; in\; [B}}{{{F}}_{{4}}}{{{]}}^{{ - }}}$. Back bonding strengthens the bond means bond length is short. So trifluoroboron has a shorter bond length than the anion. So, this option is incorrect.
So, the correct answer is Option A,C.
Note: The nitrosonium ion is the nucleophile of a very important reaction named diazotization. The nucleophile attacks an amine functional group to make oxime first and then changes into an azo group which is a very good leaving group. The boron anion given in question does not show back bonding because the boron octet is filled and there is no space, no vacant p – orbital for doing back pi bonding. That’s why it has long bond lengths and less strength.
Complete step by step answer:
First let us see the structure of the compound.

The structure shows us the number of bonds between the constituent atoms. Let’s see each options statement:
(A) According to the structure above the ${{{[B}}{{{F}}_{{4}}}{{]}}^{{ - }}}{{ has\; 4\sigma }}$ bonds and ${{N}}{{{O}}^{{ + }}}{{ has\; 1\sigma \; and \;2\pi }}$ bonds as a whole it has five sigma bonds and two pi bonds which is correct. So, option A is correct.
(B) The nitrosonium ion here has a bond order of three and nitric oxide has a bond order of ${{2}}{{.5}}$. We already know that more bonds mean higher bond order so more strength and short bond length. Since nitrosonium cation has more bond – order so less bond length than NO. So this option is incorrect.
(C) Since all the electrons in this structure are even and paired up so there is only diamagnetic nature in the compound. So, this option is correct.
(D) The ${{p\pi - p\pi }}$ back bonding occurs when the ligand is electron rich lone pairs in p – orbitals and metal in centre have vacant p – orbital then back bonding occur which is present in ${{B}}{{{F}}_{{3}}}{{ \;and\; not\; in\; [B}}{{{F}}_{{4}}}{{{]}}^{{ - }}}$. Back bonding strengthens the bond means bond length is short. So trifluoroboron has a shorter bond length than the anion. So, this option is incorrect.
So, the correct answer is Option A,C.
Note: The nitrosonium ion is the nucleophile of a very important reaction named diazotization. The nucleophile attacks an amine functional group to make oxime first and then changes into an azo group which is a very good leaving group. The boron anion given in question does not show back bonding because the boron octet is filled and there is no space, no vacant p – orbital for doing back pi bonding. That’s why it has long bond lengths and less strength.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




