
Schottky defect is:
A) Vacancy of ions.
B) Delocalization of ions.
C) Interstitial vacancy of ions.
D) Vacancy of only cations.
Answer
564.9k+ views
Hint: Solid state is the state in which the molecules are tightly bound to each other and thus have definite mass, shape and volume. But sometimes there is imperfection found in the crystalline solids. It can be point defect or the line defects.
Complete step by step answer:
There are two types of defects found in the structure. These are:
Point defect: Point defect is again classified into:
Stoichiometric defect: it can be vacancy defect or interstitial defect that can be shown as either Frenkel defect or Schottky defect that takes place in non-ionic solids.
Impurity defect: When the foreign atoms present at the vacant site of interstitial atom then impurity defect arises.
Non stoichiometric defect: it can be metal deficiency defect or metal excess defect because the ratio of anions and cations can be disturbed by removing or adding ions.
Line defect: Line defects are also called dislocations. They are two types: edge and screw defects.
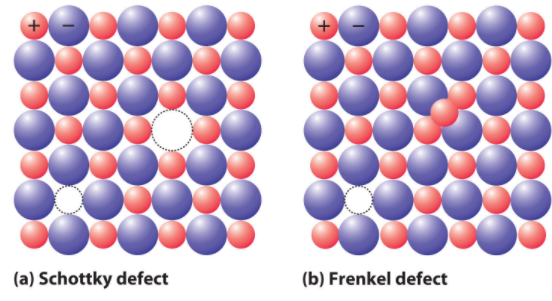
In the Schottky defect, the vacancy of ions takes place. It is a type of vacancy defect. Schottky defect reduces the density of substances and the size of anions and cations are the same.
In the Frenkel defect, displacement of ions of the crystals takes place in which the smaller ion like cation gets displaced from its normal site to an interstitial site. Thus, it is also known as a dislocation defect. It is a type of interstitial defect.
The correct option is A i.e. vacancy of ions.
Additional Information: Point defect is called so because there is an irregularity or deviation takes place from an ideal arrangement around a particular point or an atom whereas Line defects are the deviations that take place within the entire row of lattice points. These irregularities are called crystal defects.
Note: in Schottky defect, to maintain the electrical neutrality within the crystal equal number of cations and anions are missing. Thus, decreases the density of the substance. It is shown by the ionic solids having atoms of similar sizes. Examples include NaCl, KCl, CsCl and AgBr. AgBr shows both types of defect i.e. Frenkel as well as Schottky defect.
Complete step by step answer:
There are two types of defects found in the structure. These are:
Point defect: Point defect is again classified into:
Stoichiometric defect: it can be vacancy defect or interstitial defect that can be shown as either Frenkel defect or Schottky defect that takes place in non-ionic solids.
Impurity defect: When the foreign atoms present at the vacant site of interstitial atom then impurity defect arises.
Non stoichiometric defect: it can be metal deficiency defect or metal excess defect because the ratio of anions and cations can be disturbed by removing or adding ions.
Line defect: Line defects are also called dislocations. They are two types: edge and screw defects.
In the Schottky defect, the vacancy of ions takes place. It is a type of vacancy defect. Schottky defect reduces the density of substances and the size of anions and cations are the same.
In the Frenkel defect, displacement of ions of the crystals takes place in which the smaller ion like cation gets displaced from its normal site to an interstitial site. Thus, it is also known as a dislocation defect. It is a type of interstitial defect.

The correct option is A i.e. vacancy of ions.
Additional Information: Point defect is called so because there is an irregularity or deviation takes place from an ideal arrangement around a particular point or an atom whereas Line defects are the deviations that take place within the entire row of lattice points. These irregularities are called crystal defects.
Note: in Schottky defect, to maintain the electrical neutrality within the crystal equal number of cations and anions are missing. Thus, decreases the density of the substance. It is shown by the ionic solids having atoms of similar sizes. Examples include NaCl, KCl, CsCl and AgBr. AgBr shows both types of defect i.e. Frenkel as well as Schottky defect.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




