
What is Rosenmund’s reaction? What is the purpose of adding \[BaS{{O}_{4}}\]in this reaction?
Answer
595.2k+ views
Hint: Rosenmund’s reaction is an organic reaction. It is a reduction hydrogenation process. \[BaS{{O}_{4}}\] is a catalyst used in this reaction.
Complete step by step answer:
> The Rosenmund reaction is a hydrogenation process where molecular hydrogen reacts with the acyl chloride in the presence of catalyst – palladium on barium sulfate.
> The Rosenmund reaction is catalyzed by palladium on barium sulfate. The barium sulfate reduces the activity of the palladium due to its low surface area, thereby preventing over reduction. Barium sulfate reduces the activity of palladium due to its low surface area meaning it decreases the reducing power of palladium in order to prevent over-reduction of the acid.
> A poison can be added to totally deactivate the palladium catalyst. The need for deactivation arises because the subsequent aldehyde formed from the reduction of the acyl chloride will also be reduced to a primary alcohol by the system. And below reaction chlorine gets replaced by hydrogen. \[Pd/BaS{{O}_{4}}\] is the catalyst for this reaction don’t take part in chemical change. Below given is the rosenmund reaction:
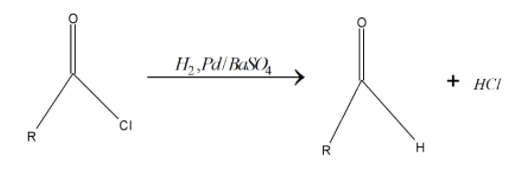
Due to the high reactivity of hydrogen gas it readily initiates a substitution in the acyl chloride, forming HCl and the required aldehyde.
Note:
Common poisons used to limit palladium activity in the Rosenmund technique are thioquinanthrene and thiourea. \[Pd/BaS{{O}_{4}}\]is known as lindlar’s catalyst.
Complete step by step answer:
> The Rosenmund reaction is a hydrogenation process where molecular hydrogen reacts with the acyl chloride in the presence of catalyst – palladium on barium sulfate.
> The Rosenmund reaction is catalyzed by palladium on barium sulfate. The barium sulfate reduces the activity of the palladium due to its low surface area, thereby preventing over reduction. Barium sulfate reduces the activity of palladium due to its low surface area meaning it decreases the reducing power of palladium in order to prevent over-reduction of the acid.
> A poison can be added to totally deactivate the palladium catalyst. The need for deactivation arises because the subsequent aldehyde formed from the reduction of the acyl chloride will also be reduced to a primary alcohol by the system. And below reaction chlorine gets replaced by hydrogen. \[Pd/BaS{{O}_{4}}\] is the catalyst for this reaction don’t take part in chemical change. Below given is the rosenmund reaction:

Due to the high reactivity of hydrogen gas it readily initiates a substitution in the acyl chloride, forming HCl and the required aldehyde.
Note:
Common poisons used to limit palladium activity in the Rosenmund technique are thioquinanthrene and thiourea. \[Pd/BaS{{O}_{4}}\]is known as lindlar’s catalyst.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




