
Rank the following compounds in order of decreasing electron density in the benzene.
Chlorobenzene (2) 4- nitrocholorbenzene (3) 2,4 – dinitrocholorbenzene (4) 2,4,6- trinitrochlorobenzene
A.1 > 2 > 3 > 4
B.1 > 3 > 2 > 4
C.3 > 1 > 4 > 2
D.4 > 3 > 2 > 1
Answer
531.6k+ views
Hint: Before solving this question, we should first know about the electron density of each benzene ring and then compare which one has greater and arrange it in decreasing order accordingly. Electron Density depends on the electronegativity of the element. The highly electronegative elements have more electrons.
Complete answer:
Greater electron density is represented by a partial negative charge whereas a partial positive charge represents less electron density.
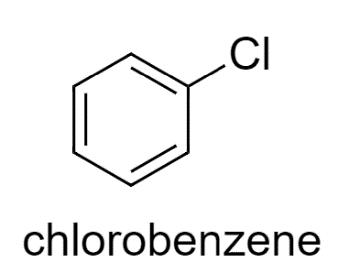
In Chlorobenzene, Cl is there which is a high electronegative element. It withdraws the electrons from the benzene ring.
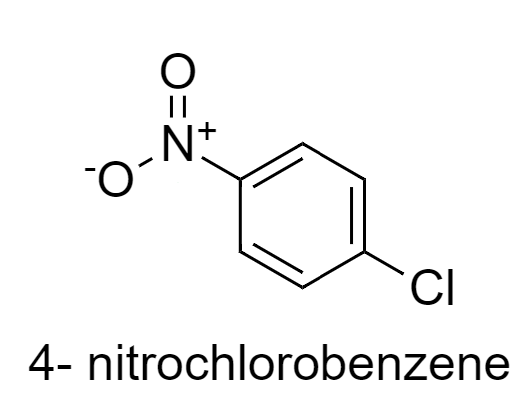
In 4-nitrochlorobenzene, Cl is there which withdraws the electrons and $N{{O}_{2}}$is also present which also has electron withdrawing property, so electron density decreases here.
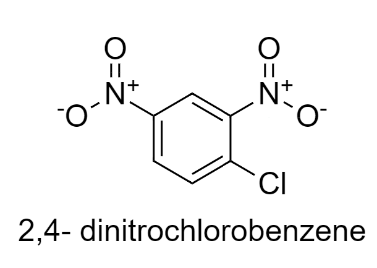
In 2,4-dinitrochlorobenzene, Cl is there which withdraws the electrons and two nitro groups are present which withdraws the electrons from the benzene ring decreasing the electron density.
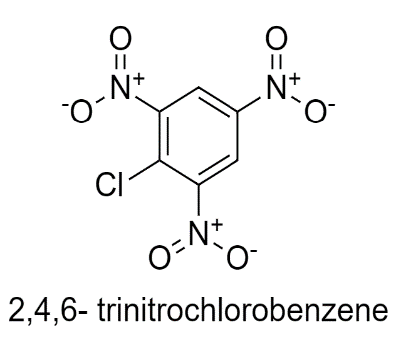
In 2,4,6- trinitrochlorobenzene, Cl is there which withdraws the electrons and three nitro groups are present which withdraws the electrons from the benzene ring decreasing the electron density.
Note:
The probability to find an electron in a certain place in a proximity of an atom or molecule, This calculation gives a quantity that is called Electron Density. Generally, The place where the electron density is high is the most prominent place for the electron to be found.
Complete answer:
Greater electron density is represented by a partial negative charge whereas a partial positive charge represents less electron density.

In Chlorobenzene, Cl is there which is a high electronegative element. It withdraws the electrons from the benzene ring.

In 4-nitrochlorobenzene, Cl is there which withdraws the electrons and $N{{O}_{2}}$is also present which also has electron withdrawing property, so electron density decreases here.

In 2,4-dinitrochlorobenzene, Cl is there which withdraws the electrons and two nitro groups are present which withdraws the electrons from the benzene ring decreasing the electron density.

In 2,4,6- trinitrochlorobenzene, Cl is there which withdraws the electrons and three nitro groups are present which withdraws the electrons from the benzene ring decreasing the electron density.
Note:
The probability to find an electron in a certain place in a proximity of an atom or molecule, This calculation gives a quantity that is called Electron Density. Generally, The place where the electron density is high is the most prominent place for the electron to be found.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




