
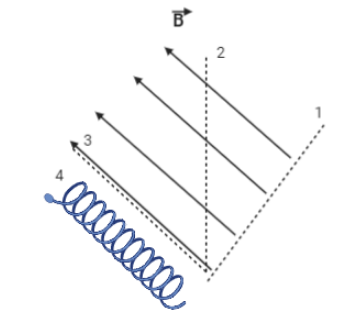
Radiation coming from a radioactive substance is passed through a cloud-chamber in which a strong constant magnetic field is switched on in the direction shown in figure. Figure shows a path followed by a few particles coming from radioactive substances (shown by dotted lines). Neglect the interaction between different particles. Tick the correct statements.
This question has multiple correct options.
A. Path 1 can be followed by $\gamma $ -radiation only
B. Path 3 can be followed by$\gamma $ -radiation only
C. Path 4 can be followed by $\alpha $ radiation only
D. Path 4 can be followed by $\beta $ radiation.

Answer
572.4k+ views
Hint:The $\alpha $-particles carry positive charge, $\beta $-particles carry negative charge and $\gamma $-particles are uncharged particles. The uncharged particles are not deflected by magnetic fields and both alpha and beta particles are deflected by both the electric and magnetic fields.
Complete answer:
A radioactive substance is emitting radiations and it is passed through a cloud-chamber in which a strong intensity of magnetic field is switched on in the direction shown by the four arrows. A cloud chamber is a device which is used to detect ionizing particles and it is also used to determine the trajectories i.e., the path followed by the different particles when it passes through the chamber. The three dotted lines and spiral shape shows the path followed by the few particles.
$\alpha $ , $\beta $ and $\gamma $ are the types of radiations. Alpha $\left( \alpha \right)$ particles are positively charged, beta $\left( \beta \right)$ particles are negatively charged and gamma $\left( \gamma \right)$ particles do not carry any charge. The alpha and beta particles are deflected by both the electric and magnetic fields as they carry charge. The deflection of alpha particles is less as compared to that of beta particles because alpha particles are more massive than beta particles and deflection of beta particles is in a direction opposite to that of alpha particle since both of them carry opposite charge.
Hence, path 1 is represented by $\gamma $ -radiation only as it is not deflected by the magnetic field i.e., it travels in a straight line. Path 4 can be followed by $\beta $-radiation because it is deflected by the magnetic field. Option C is wrong because path 4 can be followed both by $\alpha $ and $\beta $ radiations. So, the word only is not correct in option C. The $\beta $ -particle can be deflected in a straight line or in a spiral shape and it depends on the velocity of the particle.
Therefore, options A and D are correct.
Note: Kindly remember that path 1 can only be followed by $\gamma $-particles as it does not carry any charge. Thus, it would not be deflected by a magnetic field. Path 4 can be followed by both $\alpha $ and β particles because they carry charge and spiral shape depends on the speed of the particles.
Complete answer:
A radioactive substance is emitting radiations and it is passed through a cloud-chamber in which a strong intensity of magnetic field is switched on in the direction shown by the four arrows. A cloud chamber is a device which is used to detect ionizing particles and it is also used to determine the trajectories i.e., the path followed by the different particles when it passes through the chamber. The three dotted lines and spiral shape shows the path followed by the few particles.
$\alpha $ , $\beta $ and $\gamma $ are the types of radiations. Alpha $\left( \alpha \right)$ particles are positively charged, beta $\left( \beta \right)$ particles are negatively charged and gamma $\left( \gamma \right)$ particles do not carry any charge. The alpha and beta particles are deflected by both the electric and magnetic fields as they carry charge. The deflection of alpha particles is less as compared to that of beta particles because alpha particles are more massive than beta particles and deflection of beta particles is in a direction opposite to that of alpha particle since both of them carry opposite charge.
Hence, path 1 is represented by $\gamma $ -radiation only as it is not deflected by the magnetic field i.e., it travels in a straight line. Path 4 can be followed by $\beta $-radiation because it is deflected by the magnetic field. Option C is wrong because path 4 can be followed both by $\alpha $ and $\beta $ radiations. So, the word only is not correct in option C. The $\beta $ -particle can be deflected in a straight line or in a spiral shape and it depends on the velocity of the particle.
Therefore, options A and D are correct.
Note: Kindly remember that path 1 can only be followed by $\gamma $-particles as it does not carry any charge. Thus, it would not be deflected by a magnetic field. Path 4 can be followed by both $\alpha $ and β particles because they carry charge and spiral shape depends on the speed of the particles.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




