
Quartz, the mineral used in watches, is made of silicon and oxygen atoms joined in a network arrangement that is similar to:
a.) Diamond
b.) Iron metal
c.) Graphite used in pencils
d.) Oxygen in the air
Answer
573.3k+ views
Hint: Quartz is a crystalline mineral composed of silicon and oxygen. The atoms are linked in a vast network of silicon-oxygen tetrahedral with each oxygen atom being shared between two tetrahedral with an overall chemical formula of $Si{{O}_{2}}$.
Complete Solution :
Below is the picture depiction of quartz crystal structure:
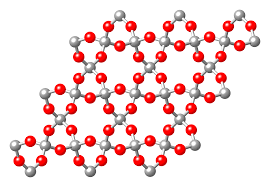
- Here you can clearly see that each silicon atom is sharing its electrons with 4 oxygen atoms and each oxygen atom is shared between two tetrahedral and it is having network like structure.
- If we compare this structure with the structures of the compounds given in the options then you will notice that there are two network like solids present among them and that are diamond and graphite.
- We know that diamond has a structure where one carbon is tetrahedrally attached to four other carbon atoms. Its structure is shown below:
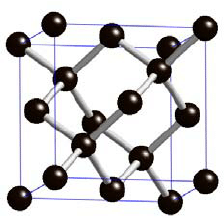
So clearly the structure of Quartz resembles the structure of diamond.
So, the correct answer is “Option A”.
Additional Information:
Quartz crystals maintain a precise frequency standard, which helps to regulate the movement of a watch or clock, thus making the timepieces very accurate. Quartz is also used in radios, microprocessors, and many other technological and industrial applications.
Note: Quartz is classified as one of the silicates with chemical composition of 46.7% Silicon and 53.3% oxygen. It is extremely common and can be found in many types of igneous, sedimentary and metamorphic rocks. Quartz occurs in virtually every color. Common colors are clear, white, gray, purple, yellow, brown, black, pink, green, red.
Complete Solution :
Below is the picture depiction of quartz crystal structure:

- Here you can clearly see that each silicon atom is sharing its electrons with 4 oxygen atoms and each oxygen atom is shared between two tetrahedral and it is having network like structure.
- If we compare this structure with the structures of the compounds given in the options then you will notice that there are two network like solids present among them and that are diamond and graphite.
- We know that diamond has a structure where one carbon is tetrahedrally attached to four other carbon atoms. Its structure is shown below:

So clearly the structure of Quartz resembles the structure of diamond.
So, the correct answer is “Option A”.
Additional Information:
Quartz crystals maintain a precise frequency standard, which helps to regulate the movement of a watch or clock, thus making the timepieces very accurate. Quartz is also used in radios, microprocessors, and many other technological and industrial applications.
Note: Quartz is classified as one of the silicates with chemical composition of 46.7% Silicon and 53.3% oxygen. It is extremely common and can be found in many types of igneous, sedimentary and metamorphic rocks. Quartz occurs in virtually every color. Common colors are clear, white, gray, purple, yellow, brown, black, pink, green, red.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




