
How is propanone converted into-
1.Propan-2-ol
2.2-Methylpropan-2-ol
Answer
589.5k+ views
Hint: We know that ketone comprises a carbonyl group, a functional group with a carbon-oxygen double bond. In ketones, the carbonyl group is linked to two carbon atoms.
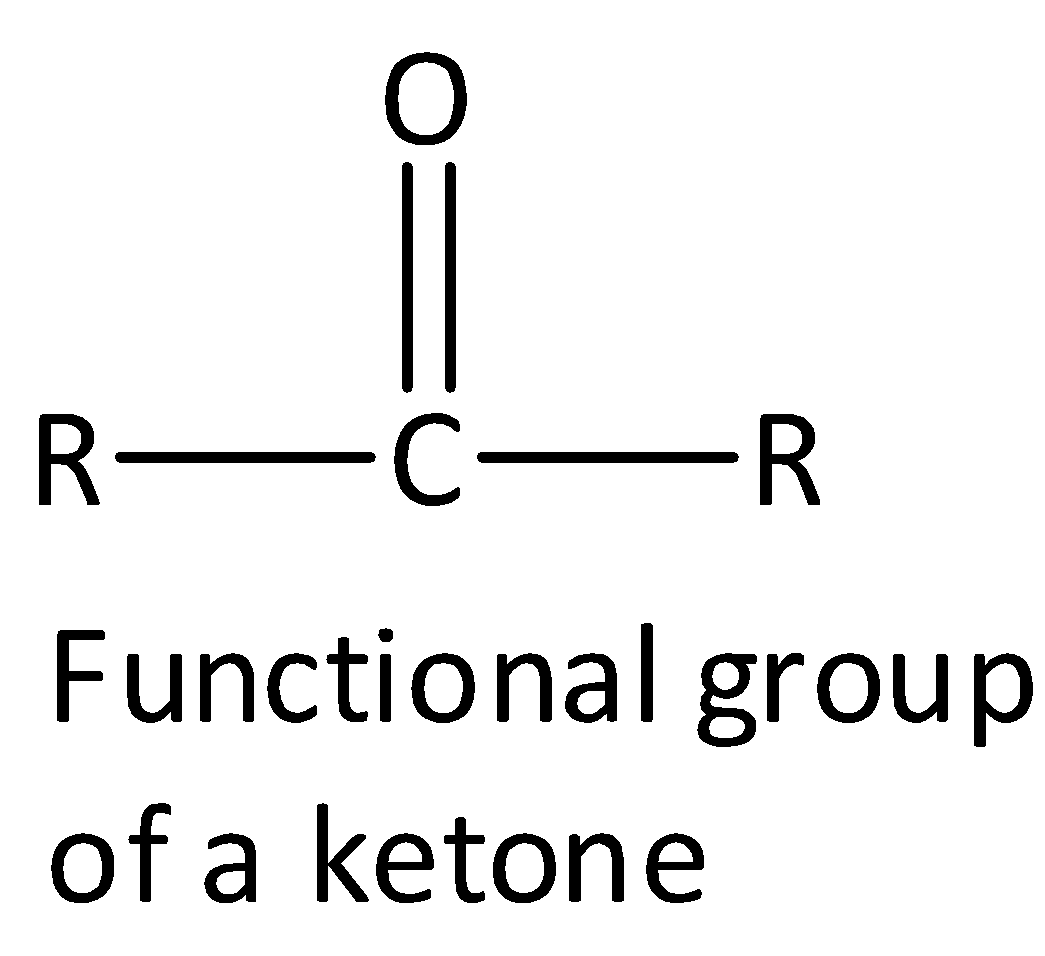
R is the alkyl group.
Aldehydes and ketones are prepared by the oxidation of the corresponding alcohols.
The product of primary alcohol oxidation is an aldehyde and the product of secondary alcohol is a ketone. We have to know that tertiary alcohols do not undergo oxidation under the conditions normally used.
Complete step by step answer:
Reduction in organic molecules:
In the organic system, reduction is known as the gain of hydrogen or loss of oxygen.
Reduction in aldehydes (or) ketones:
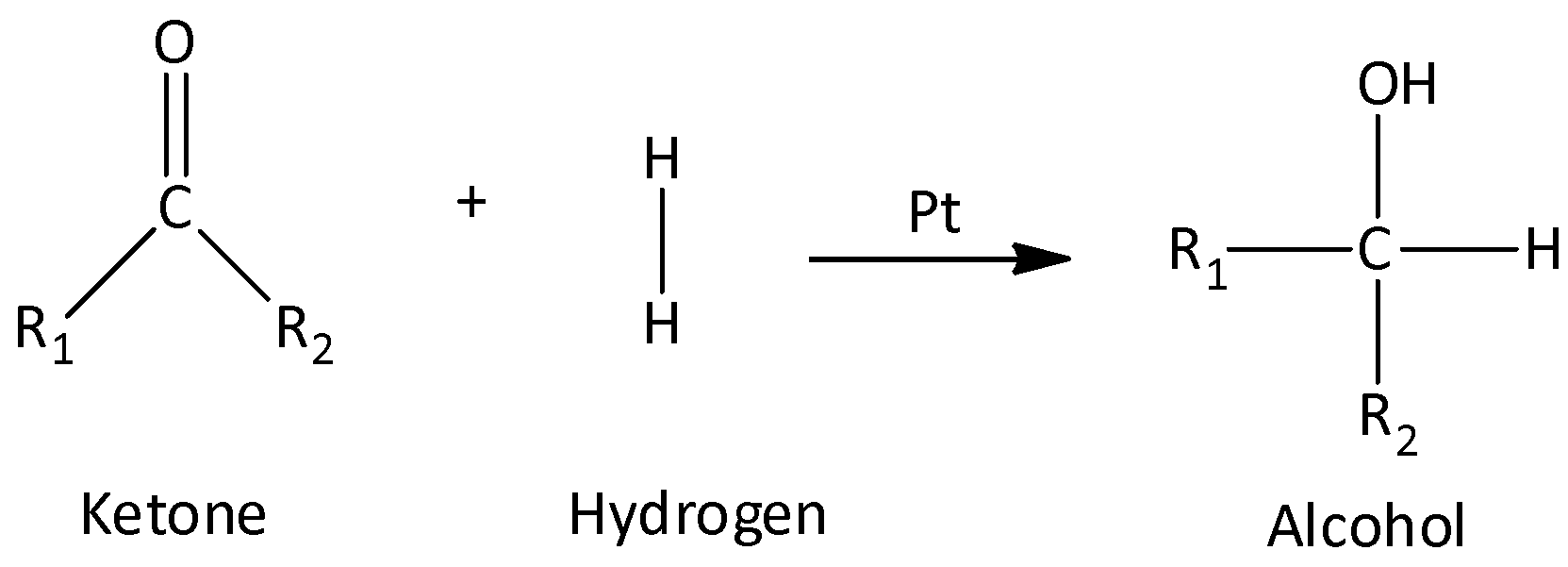
Hydrogenation is the method of reducing an aldehyde or ketone. The carbonyl compound is reacted with hydrogen gas in presence of catalysts such as nickel, platinum or palladium metal.
The hydrogenation of ketone produces a secondary alcohol.
The general reaction is,
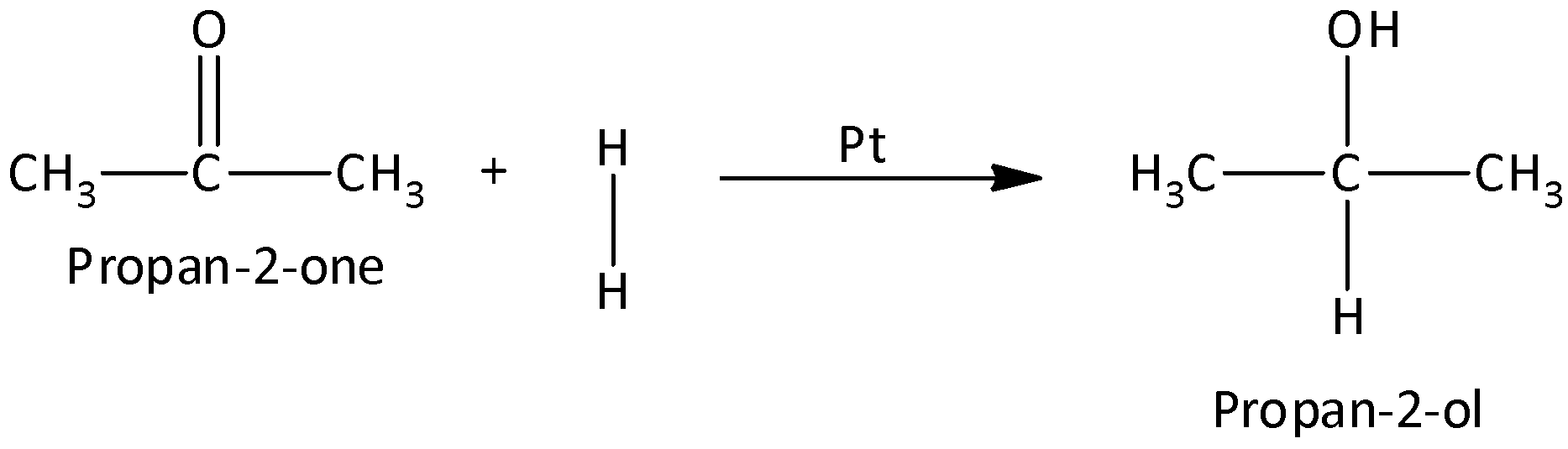
We can convert propanone to propan-2-ol by reducing ketones. Reduction of ketones gives secondary alcohol. Reduction of ketones can be done by hydrogenation (or) using Grignard Reagent.
Let us now reduce propanone to propan-2-ol by hydrogenation.
The reduction of propane in the presence of catalyst platinum along with hydrogen gives the product propan-2-ol.
We can write the chemical reaction as,
We can also use catalysts such as $LiAl{H_4}$, $THF$.
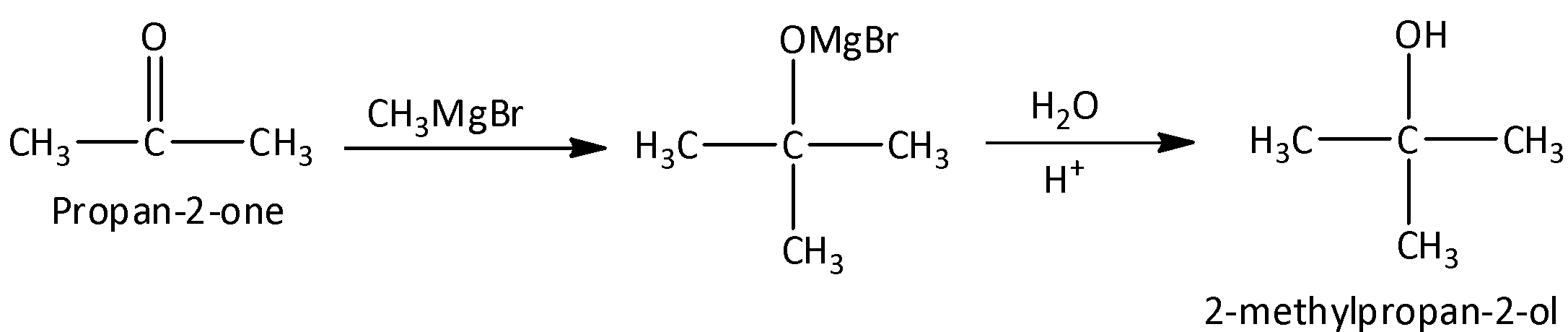
(ii) Reduction of propanone to 2-methylpropan-2-ol
We can reduce propanone to 2-methylpropan-2-ol using Grignard Reagent. We can react with propanone with methylmagnesium bromide to form an intermediate and the intermediate is hydrolyzed to form 2-methylpropan-2-ol. We can write the chemical reaction as,
Note:
We know aldehydes and ketones cannot form hydrogen bonds to one another, but they can form intermolecular hydrogen bonds with water. As result, the smaller members (five or fewer carbon atoms) are soluble in water. Aldehydes and ketones have lower boiling points when compared to carboxylic acid, representing the presence of weak intermolecular dipole-dipole forces. They do not form hydrogen bonds with other aldehydes or ketones because there is no oxygen-hydrogen bond in the carbonyl group. Due to weaker intermolecular hydrogen bonding in aldehydes and weaker dipole-dipole attractions, they have lower boiling points compared to carboxylic acids.

R is the alkyl group.
Aldehydes and ketones are prepared by the oxidation of the corresponding alcohols.
The product of primary alcohol oxidation is an aldehyde and the product of secondary alcohol is a ketone. We have to know that tertiary alcohols do not undergo oxidation under the conditions normally used.
Complete step by step answer:
Reduction in organic molecules:
In the organic system, reduction is known as the gain of hydrogen or loss of oxygen.
Reduction in aldehydes (or) ketones:
Hydrogenation is the method of reducing an aldehyde or ketone. The carbonyl compound is reacted with hydrogen gas in presence of catalysts such as nickel, platinum or palladium metal.
The hydrogenation of ketone produces a secondary alcohol.
The general reaction is,

- (i) Reduction of propanone to propan-2-ol
We can convert propanone to propan-2-ol by reducing ketones. Reduction of ketones gives secondary alcohol. Reduction of ketones can be done by hydrogenation (or) using Grignard Reagent.
Let us now reduce propanone to propan-2-ol by hydrogenation.
The reduction of propane in the presence of catalyst platinum along with hydrogen gives the product propan-2-ol.
We can write the chemical reaction as,

We can also use catalysts such as $LiAl{H_4}$, $THF$.
We can reduce propanone to 2-methylpropan-2-ol using Grignard Reagent. We can react with propanone with methylmagnesium bromide to form an intermediate and the intermediate is hydrolyzed to form 2-methylpropan-2-ol. We can write the chemical reaction as,

Note:
We know aldehydes and ketones cannot form hydrogen bonds to one another, but they can form intermolecular hydrogen bonds with water. As result, the smaller members (five or fewer carbon atoms) are soluble in water. Aldehydes and ketones have lower boiling points when compared to carboxylic acid, representing the presence of weak intermolecular dipole-dipole forces. They do not form hydrogen bonds with other aldehydes or ketones because there is no oxygen-hydrogen bond in the carbonyl group. Due to weaker intermolecular hydrogen bonding in aldehydes and weaker dipole-dipole attractions, they have lower boiling points compared to carboxylic acids.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




