
What is the product of reaction and its formation of selectivity
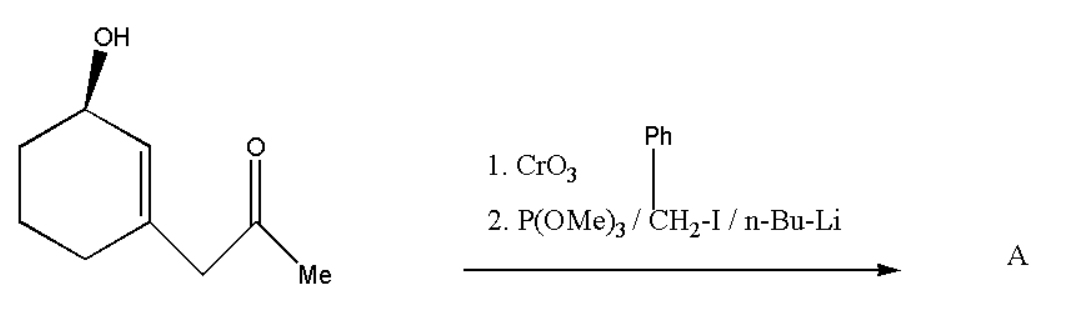

Answer
493.2k+ views
Hint: The reaction is an example of the Wittig reaction. The given ketone will react with triphenyl phosphonium ylide which is also known as Wittig reagent. Firstly we will make this Wittig reagent and then it will attack on the ketonic group of the compound and hence after rearrangement of atoms we will get our desired product A.
Complete answer:
Here the given compound consists of both alcohol and carbonyl. But when we add \[Cr{O_3}\] to the compound then oxidation of alcohol takes place and we get another carbonyl group. \[Cr{O_3}\] is a strong oxidising agent, therefore it will convert alcohol directly into a carbonyl group.
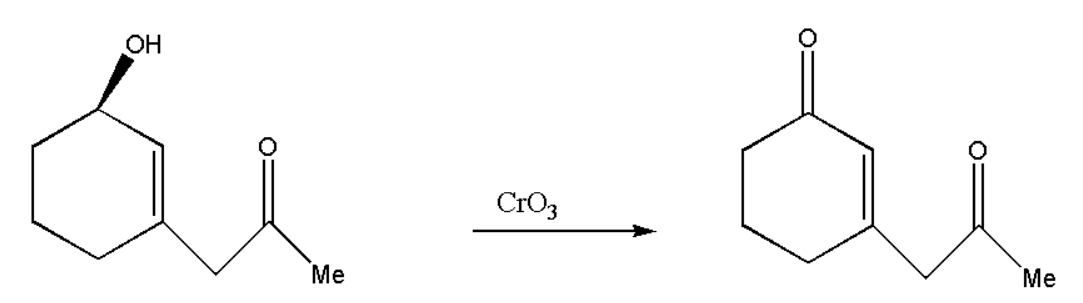
Now we will take the reagent and make a finalized product which will react with our oxidized product. The reaction between the reagents can be represented in steps ass,
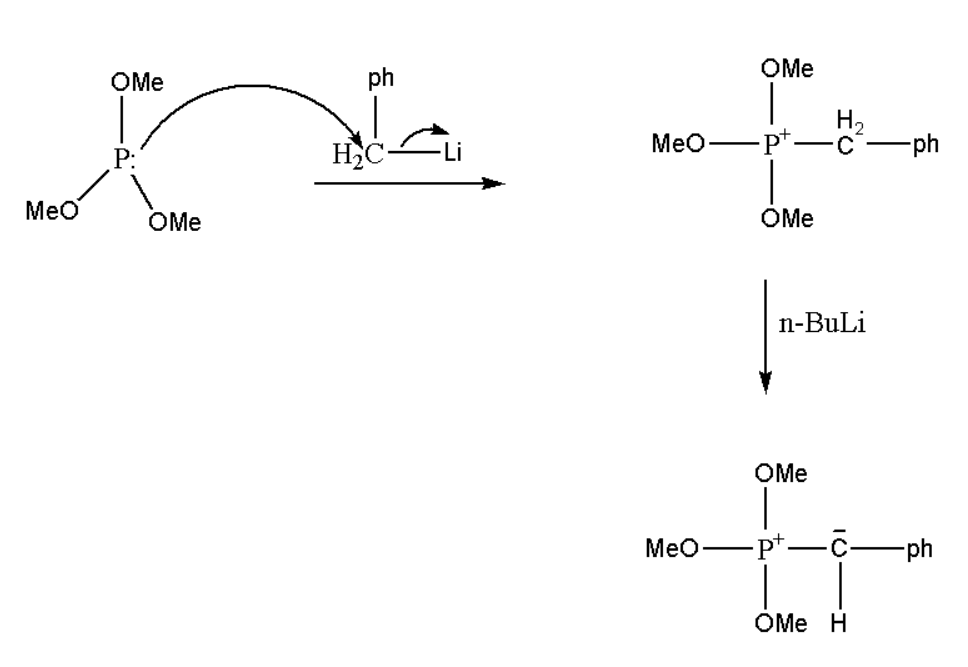
Step \[1.\] Tri-methoxy phosphorus will react with phenyl methyl iodide and form an intermediate.
Step \[2.\] This intermediate will further react with the base known as \[n - BuLi\] and it will develop a negative charge on the carbon atom.
Therefore we get ylide which will react with our oxidized product and will form alkene by rearrangement of atoms. This reagent will attack on that carbon which is more electron deficient, thus which decides its selectivity of the formed product.
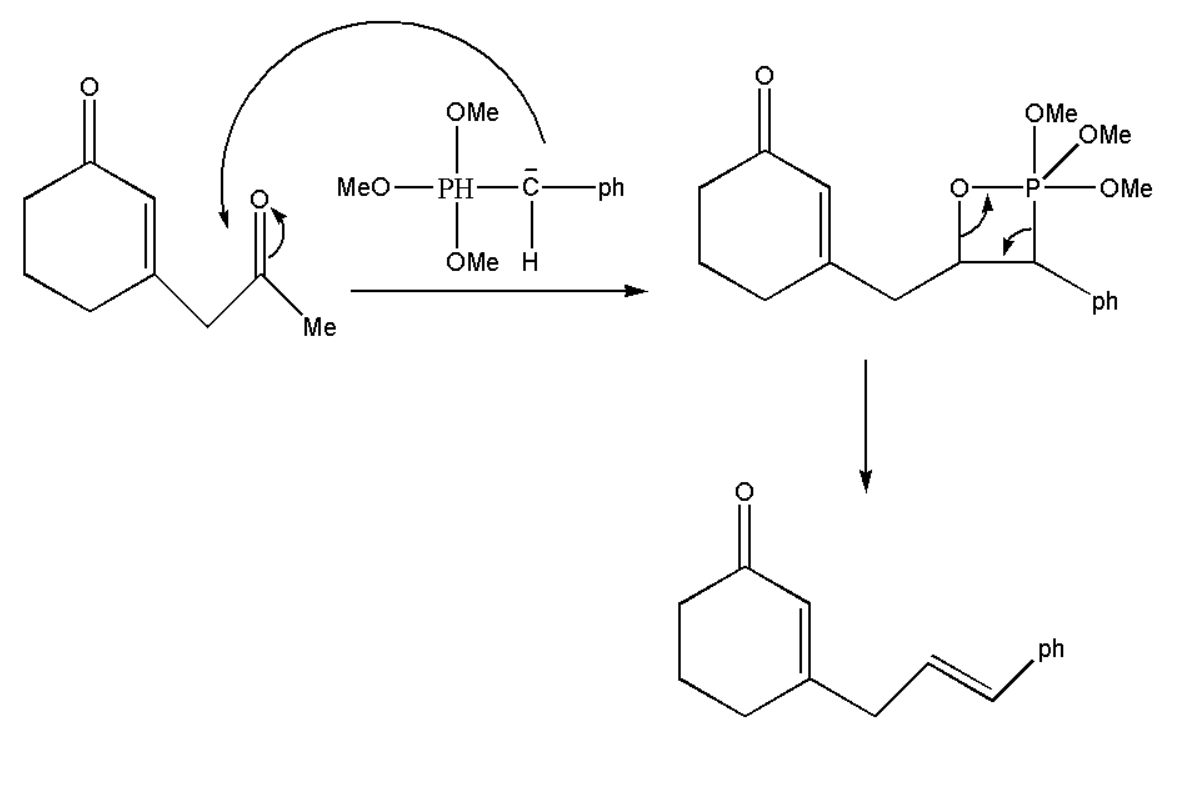
Thus we get our product A which is an alkene.
Note:
The reagent will attack on that carbonyl group which is more deficient of electrons which is also known as selectivity principle. The Wittig reaction is used for manufacturing the alkene from ketones or aldehydes. We must take care of rearrangement of atoms.
Complete answer:
Here the given compound consists of both alcohol and carbonyl. But when we add \[Cr{O_3}\] to the compound then oxidation of alcohol takes place and we get another carbonyl group. \[Cr{O_3}\] is a strong oxidising agent, therefore it will convert alcohol directly into a carbonyl group.

Now we will take the reagent and make a finalized product which will react with our oxidized product. The reaction between the reagents can be represented in steps ass,
Step \[1.\] Tri-methoxy phosphorus will react with phenyl methyl iodide and form an intermediate.
Step \[2.\] This intermediate will further react with the base known as \[n - BuLi\] and it will develop a negative charge on the carbon atom.

Therefore we get ylide which will react with our oxidized product and will form alkene by rearrangement of atoms. This reagent will attack on that carbon which is more electron deficient, thus which decides its selectivity of the formed product.

Thus we get our product A which is an alkene.
Note:
The reagent will attack on that carbonyl group which is more deficient of electrons which is also known as selectivity principle. The Wittig reaction is used for manufacturing the alkene from ketones or aldehydes. We must take care of rearrangement of atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




