
How will you prepare the following compounds from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom:
i. Methyl benzoate ii. m-Nitrobenzoic acid
iii. p-Nitrobenzoic acid iv. Phenylacetic acid
v. p-Nitrobenzaldehyde
Answer
584.7k+ views
Hint: Benzene is an aromatic compound which shows the characteristic reaction of electrophilic aromatic substitution reactions. This is because the reaction is initiated by an electrophile.
Complete step by step solution:
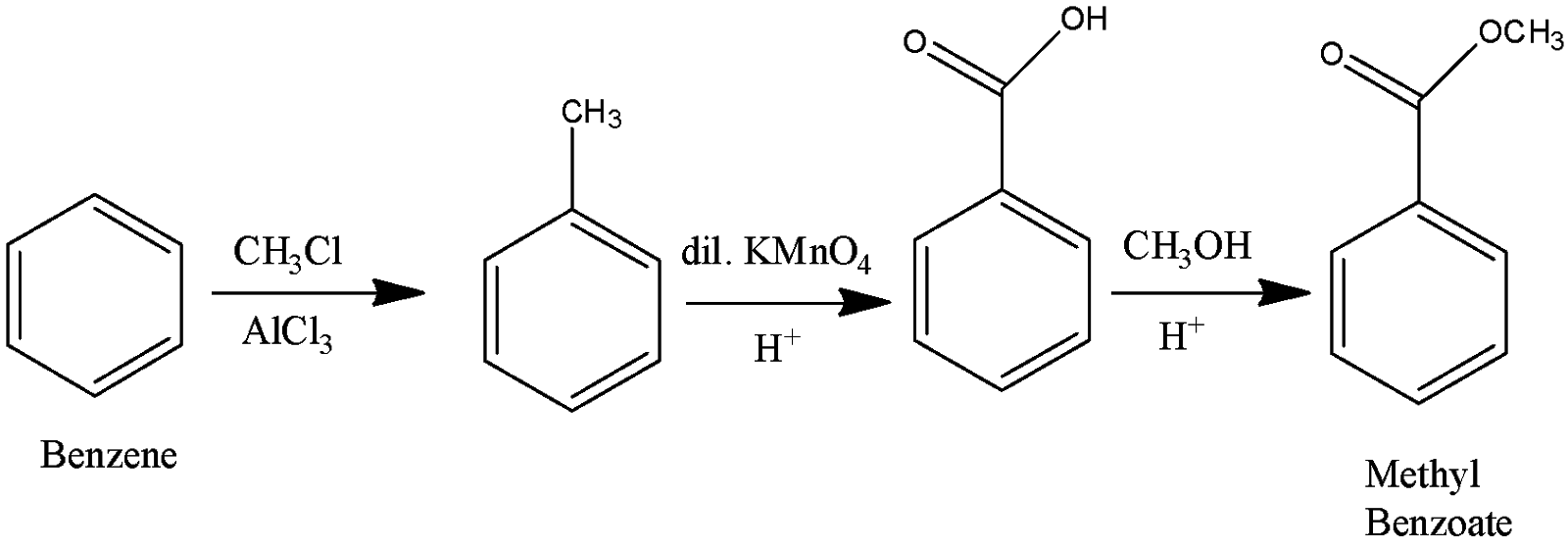
(i) Methyl benzoate is the ester of benzene. It can easily be prepared after preparing benzoic acid. So, we can form benzoic acid by Friedel Craft alkylation and then form an ester, we can just oxidize the formed acid to get methyl benzoate. The process is called esterification.
(ii) One of the many possible ways to prepare m-nitrobenzoic acid is by first forming acetophenone by Friedel Craft acylation and then form nitroacetophenone by nitration of the compound. Oxidation of the compound gives us our desired product.

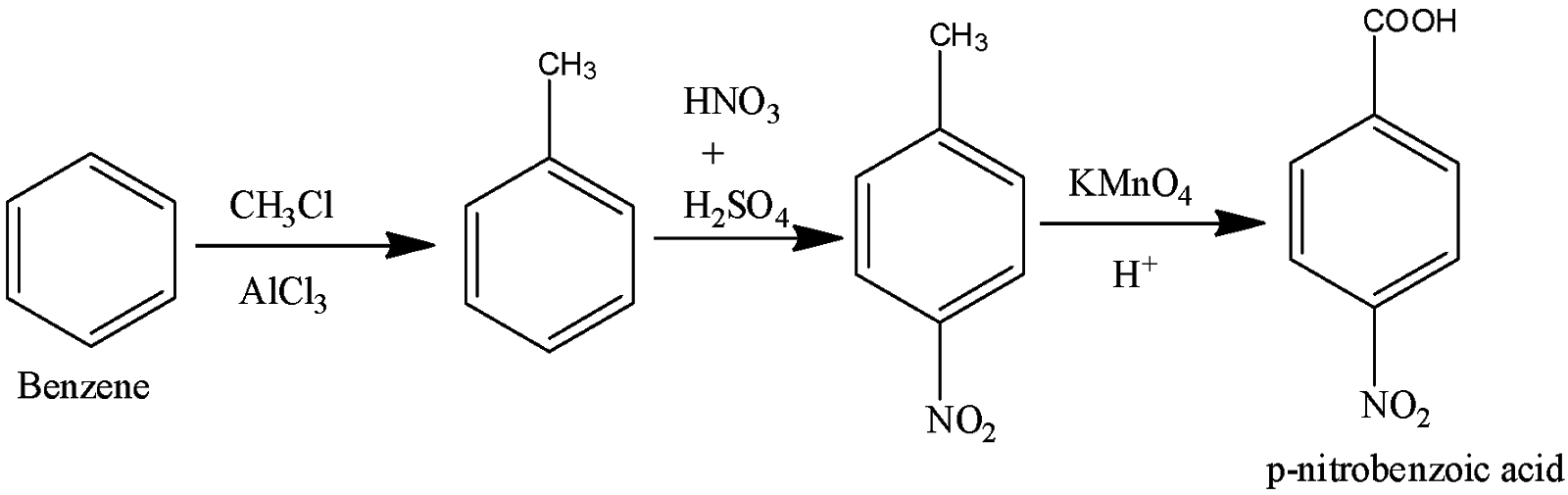
(iii) To form p-nitrobenzoic acid, first, perform Friedel Craft alkylation to get toluene and then form p-nitrotoluene by nitration. Minor products can be eliminated by filtration. Oxidation of the compound gives us p-nitrobenzoic acid.
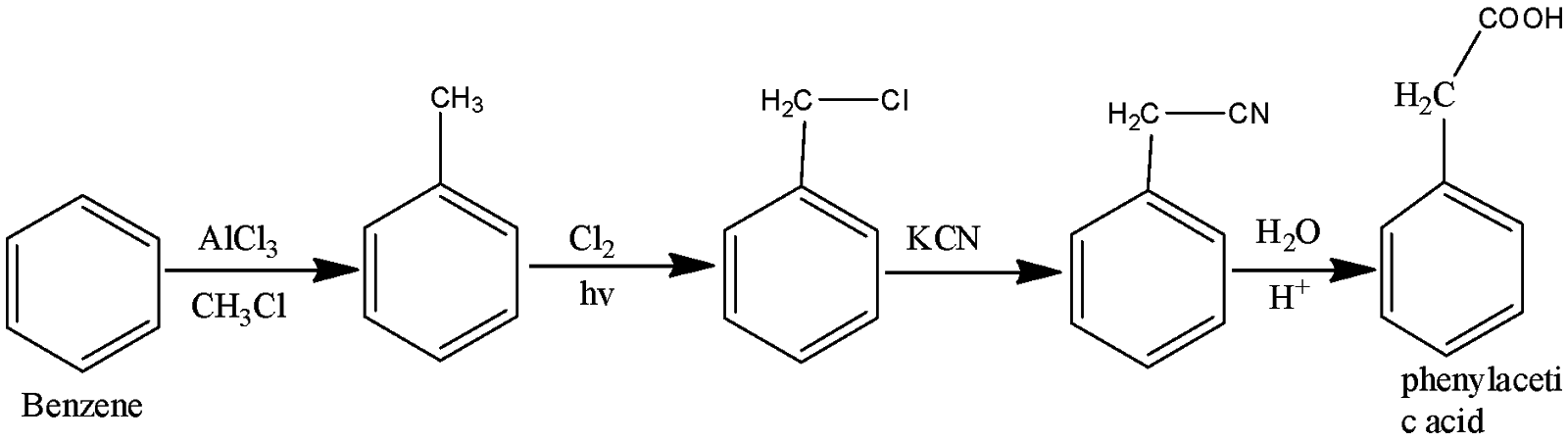
(iv) To form phenylacetic acid, first form toluene by alkylation. Then perform side chain chlorination to form benzyl chloride which can be easily replaced to benzyl cyanide by KCN. Hydrolysis in an acidic medium gives us our required product.
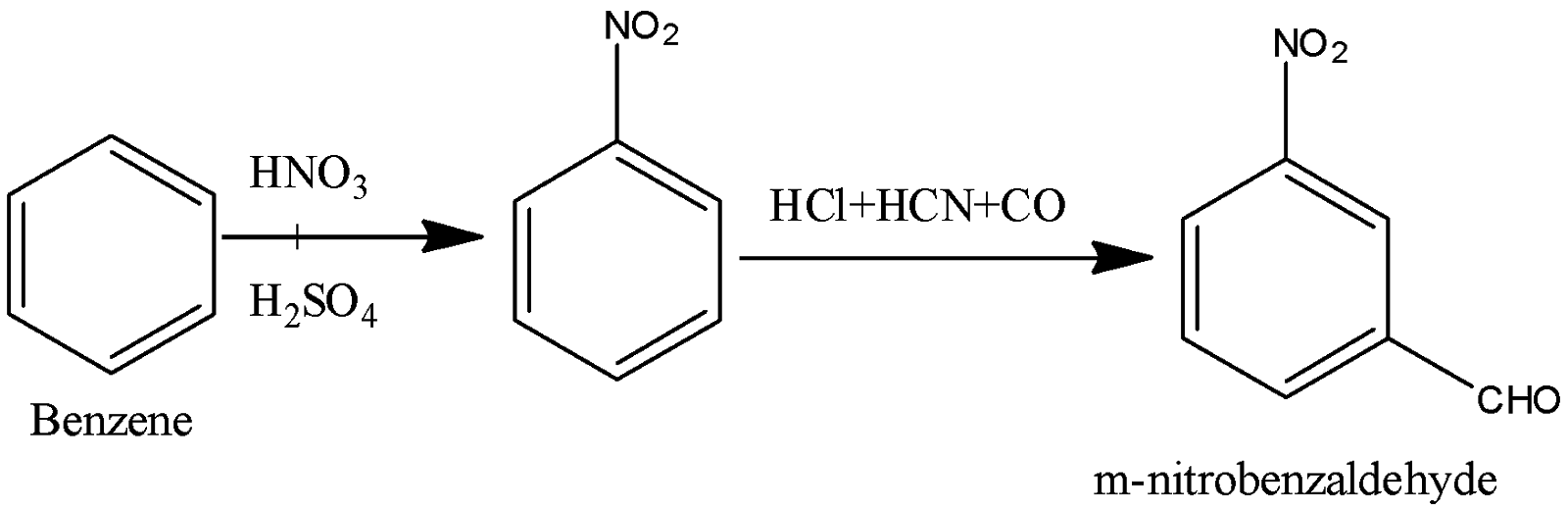
(v) Nitration of benzene gives us nitrobenzene. Performing Gaterman aldehyde synthesis using CO, HCl and HCN gives us p-nitrobenzaldehyde.
Note: Benzene is not able to show nucleophilic substitution generally because nucleophilic substitution results in the formation of non-aromatic compounds which are less stable than aromatic compounds.
Complete step by step solution:
(i) Methyl benzoate is the ester of benzene. It can easily be prepared after preparing benzoic acid. So, we can form benzoic acid by Friedel Craft alkylation and then form an ester, we can just oxidize the formed acid to get methyl benzoate. The process is called esterification.

(ii) One of the many possible ways to prepare m-nitrobenzoic acid is by first forming acetophenone by Friedel Craft acylation and then form nitroacetophenone by nitration of the compound. Oxidation of the compound gives us our desired product.

(iii) To form p-nitrobenzoic acid, first, perform Friedel Craft alkylation to get toluene and then form p-nitrotoluene by nitration. Minor products can be eliminated by filtration. Oxidation of the compound gives us p-nitrobenzoic acid.

(iv) To form phenylacetic acid, first form toluene by alkylation. Then perform side chain chlorination to form benzyl chloride which can be easily replaced to benzyl cyanide by KCN. Hydrolysis in an acidic medium gives us our required product.

(v) Nitration of benzene gives us nitrobenzene. Performing Gaterman aldehyde synthesis using CO, HCl and HCN gives us p-nitrobenzaldehyde.

Note: Benzene is not able to show nucleophilic substitution generally because nucleophilic substitution results in the formation of non-aromatic compounds which are less stable than aromatic compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




