
How can you prepare ethyl acetate from acetic acid?
Answer
558.9k+ views
Hint: Ethyl acetate is an ester of acetic acid and ethanol, with chemical formula $[C{H_3}COOC{H_2}C{H_3}]$. It is a colourless compound with sweet and fruity odour. It is a volatile compound. It is most commonly found ester in fruits.
Acetic acid or ethanoic acid is a carboxylic acid and is a constituent of vinegar. The chemical formula of acetic acid is $[C{H_3}COOH]$.
Complete step by step answer:
As we know, ethyl acetate is an ester. Given below is the structure of ethyl acetate.
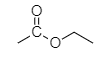
To prepare ethyl acetate from acetic acid, esterification of acetic acid should be carried out.
When acetic acid reacts with ethanol in the presence of sulphuric acid, ethyl acetate is produced through the process of esterification. Esterification is the chemical reaction between carboxylic acid and an alcohol to produce an ester.
In this chemical reaction, ${H_2}S{O_4}$ acts as a dehydrating agent and eliminates water molecules to produce ester. The process of esterification is a reversible reaction and is quite a slow process. The ${H_2}S{O_4}$ also acts as a catalyst in this reaction.
The chemical reaction for the formation of ethyl acetate from acetic acid is shown below.
$C{H_3}COOH + C{H_3}C{H_2}OH \rightleftharpoons C{H_3}COOC{H_3}C{H_2} + {H_2}O$
The reaction is carried out by heating the reactants in the presence of ${H_2}S{O_4}$.
Additional information:
Ethyl acetate is an ester and esters are extensively used in fragrance, food flavouring and in cosmetics due to its pleasant smell. Esters are also used as an organic solvent. The polymer of ester that is polyester is used in the production of plastic.
Note: The esterification reaction involves combining carboxylic acid and an alcohol in presence of ${H_2}S{O_4}$ to obtain an ester. Remember that the alcohol mentioned here should be a primary alcohol.
Esterification can also take place by reacting an acid anhydride with an alcohol or the reaction between an acid chloride with an alcohol. Here, the alcohol mentioned is the primary alcohol.
Acetic acid or ethanoic acid is a carboxylic acid and is a constituent of vinegar. The chemical formula of acetic acid is $[C{H_3}COOH]$.
Complete step by step answer:
As we know, ethyl acetate is an ester. Given below is the structure of ethyl acetate.

To prepare ethyl acetate from acetic acid, esterification of acetic acid should be carried out.
When acetic acid reacts with ethanol in the presence of sulphuric acid, ethyl acetate is produced through the process of esterification. Esterification is the chemical reaction between carboxylic acid and an alcohol to produce an ester.
In this chemical reaction, ${H_2}S{O_4}$ acts as a dehydrating agent and eliminates water molecules to produce ester. The process of esterification is a reversible reaction and is quite a slow process. The ${H_2}S{O_4}$ also acts as a catalyst in this reaction.
The chemical reaction for the formation of ethyl acetate from acetic acid is shown below.
$C{H_3}COOH + C{H_3}C{H_2}OH \rightleftharpoons C{H_3}COOC{H_3}C{H_2} + {H_2}O$
The reaction is carried out by heating the reactants in the presence of ${H_2}S{O_4}$.
Additional information:
Ethyl acetate is an ester and esters are extensively used in fragrance, food flavouring and in cosmetics due to its pleasant smell. Esters are also used as an organic solvent. The polymer of ester that is polyester is used in the production of plastic.
Note: The esterification reaction involves combining carboxylic acid and an alcohol in presence of ${H_2}S{O_4}$ to obtain an ester. Remember that the alcohol mentioned here should be a primary alcohol.
Esterification can also take place by reacting an acid anhydride with an alcohol or the reaction between an acid chloride with an alcohol. Here, the alcohol mentioned is the primary alcohol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




