
How is phenol converted to salicylic acid?
Answer
510.6k+ views
Hint :Benzene attached to a hydroxyl functional group is called phenol. Salicylic acid is a derivative of phenol itself with an additional carboxylic acid functional group attached to a position ortho to the hydroxyl group of phenol.
Complete Step By Step Answer:
Salicylic acid is an important industrial organic compound that can be derived from phenol through two different types of reactions. It contains the same structure as that of phenol with an additional carboxylic acid group $ (COOH) $ placed at the ortho position (adjacent position) of the hydroxyl group of phenol.
The two methods of converting phenol to salicylic acid are as follows:
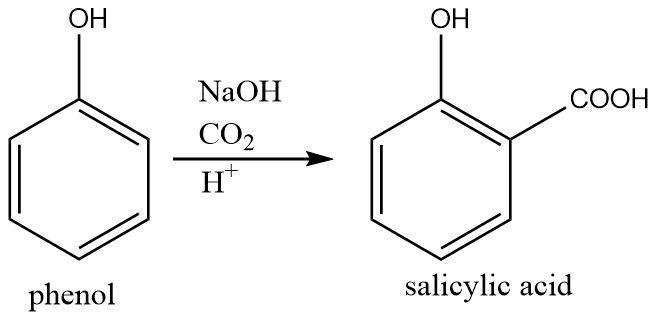
Kolbe-Schmitt reaction: This reaction involves the conversion of acidic phenol into a phenoxide ion in presence of sodium hydroxide base. This is followed by the attack of phenoxide ion of the electrophilic carbon dioxide gas resulting in a carboxylate group getting attached at the ortho position. The reaction ends with a mild acidic hydrolysis that gives salicylic acid.
Thus it is a base facilitated carboxylation of phenol.
The overall reaction can be written as follows:
$ {\text{phenol}}\xrightarrow[{{H^ + }}]{\begin{subarray}{l}
NaOH \\
C{O_2}
\end{subarray} }{\text{salicylic acid}} $
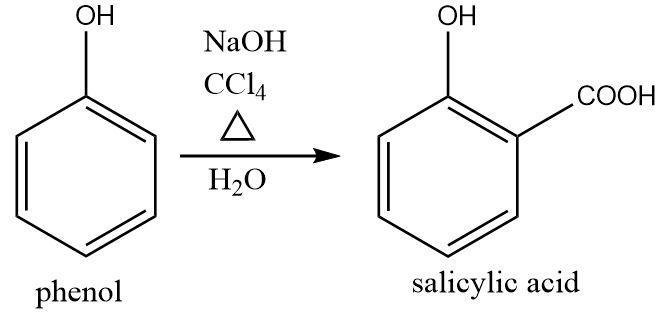
Reimer–Tiemann reaction: This reaction involves the conversion of phenol to salicylic acid by treatment of phenol at high temperatures with carbon tetrachloride in the presence of an alkali followed by mild hydrolysis.
The reaction can be written as follows:
$ {\text{phenol}}\xrightarrow[{{H_2}O}]{\begin{subarray}{l}
NaOH \\
CC{l_4}
\end{subarray} }{\text{salicylic acid}} $
Note :
The Reimer–Tiemann reaction can be performed with both chloroform as well as carbon tetrachloride. The reaction in the presence of chloroform as a reagent gives salicylaldehyde, however that in the presence of carbon tetrachloride gives salicylic acid as the desired product.
Complete Step By Step Answer:
Salicylic acid is an important industrial organic compound that can be derived from phenol through two different types of reactions. It contains the same structure as that of phenol with an additional carboxylic acid group $ (COOH) $ placed at the ortho position (adjacent position) of the hydroxyl group of phenol.
The two methods of converting phenol to salicylic acid are as follows:
Kolbe-Schmitt reaction: This reaction involves the conversion of acidic phenol into a phenoxide ion in presence of sodium hydroxide base. This is followed by the attack of phenoxide ion of the electrophilic carbon dioxide gas resulting in a carboxylate group getting attached at the ortho position. The reaction ends with a mild acidic hydrolysis that gives salicylic acid.
Thus it is a base facilitated carboxylation of phenol.
The overall reaction can be written as follows:
$ {\text{phenol}}\xrightarrow[{{H^ + }}]{\begin{subarray}{l}
NaOH \\
C{O_2}
\end{subarray} }{\text{salicylic acid}} $

Reimer–Tiemann reaction: This reaction involves the conversion of phenol to salicylic acid by treatment of phenol at high temperatures with carbon tetrachloride in the presence of an alkali followed by mild hydrolysis.
The reaction can be written as follows:
$ {\text{phenol}}\xrightarrow[{{H_2}O}]{\begin{subarray}{l}
NaOH \\
CC{l_4}
\end{subarray} }{\text{salicylic acid}} $

Note :
The Reimer–Tiemann reaction can be performed with both chloroform as well as carbon tetrachloride. The reaction in the presence of chloroform as a reagent gives salicylaldehyde, however that in the presence of carbon tetrachloride gives salicylic acid as the desired product.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




