
How is phenol (carbolic acid) prepared from Rasching’s process?
Answer
542.1k+ views
Hint :Generally we call it the Raschig - Hooker process. This process is used for production of phenol. We use Rasching’s process for the preparation of chlorobenzene. After that we convert the obtained chlorobenzene to phenol by hydrolysis that is by adding water.
Complete step by step solution:
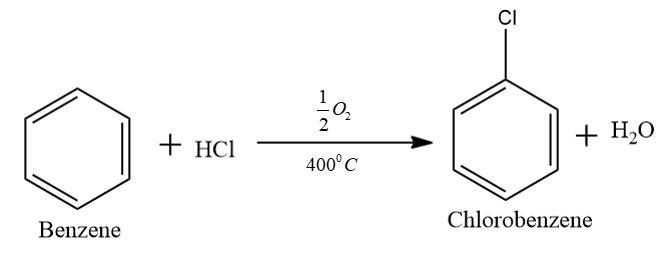
In the first step we convert the benzene into chlorobenzene. That is when benzene reacts with hydrochloric acid in the presence of oxygen we will get chlorobenzene. In this step we use either copper or iron chloride catalyst and we expose the material to air at $ {400^0}C $ .
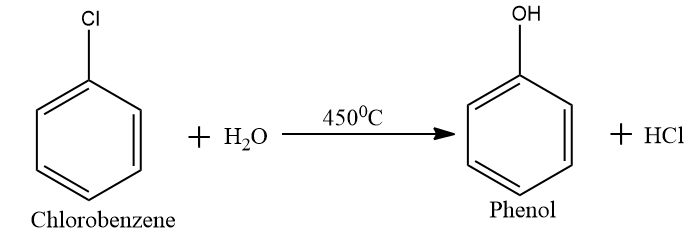
In step two we convert the obtained chlorobenzene into phenol by hydrolyses. In this step we expose the material (chlorobenzene) to a steam at $ {450^0}C $
Additional Information:
Phenol is a useful precursor to a huge collection of drugs, most notably aspirin but also several herbicides and pharmaceutical drugs. Phenol by-products have been used in the making of cosmetics including hair colouring, sunscreens and in skin lightening preparations.
Note:
As we can see that the reaction is taking place at high temperature in a very acidic environment with hydrogen chloride vapour and therefore the industrial setting must use highly corrosion resistant equipment for the reaction. However we can prepare phenol by many methods. That is by Dow process, preparation of phenol from cumene or from haloarenes or from benzene sulphonic acid, Air oxidation of cumene and many others.
Complete step by step solution:
In the first step we convert the benzene into chlorobenzene. That is when benzene reacts with hydrochloric acid in the presence of oxygen we will get chlorobenzene. In this step we use either copper or iron chloride catalyst and we expose the material to air at $ {400^0}C $ .

In step two we convert the obtained chlorobenzene into phenol by hydrolyses. In this step we expose the material (chlorobenzene) to a steam at $ {450^0}C $

Additional Information:
Phenol is a useful precursor to a huge collection of drugs, most notably aspirin but also several herbicides and pharmaceutical drugs. Phenol by-products have been used in the making of cosmetics including hair colouring, sunscreens and in skin lightening preparations.
Note:
As we can see that the reaction is taking place at high temperature in a very acidic environment with hydrogen chloride vapour and therefore the industrial setting must use highly corrosion resistant equipment for the reaction. However we can prepare phenol by many methods. That is by Dow process, preparation of phenol from cumene or from haloarenes or from benzene sulphonic acid, Air oxidation of cumene and many others.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




