
Why is para product major and not ortho?
Answer
508.8k+ views
Hint : Whenever there are groups present next to each other they produce hindrance which is known as a steric hindrance. Ortho products have steric hindrance whereas there is no such thing in para products and it is considered as major. These direct the incoming atom towards the ortho and para positions.
Complete Step By Step Answer:
First, let us understand in detail about ortho and para. These are the prefixes used to indicate the positions of a substituent on a benzene derivative or even benzene. In ortho position, the substituents occupy the positions next to each other (position $1 $ and position $2 $). In the case of para position, the substituent occupies the opposite ends (position $1 $ and position $4 $)
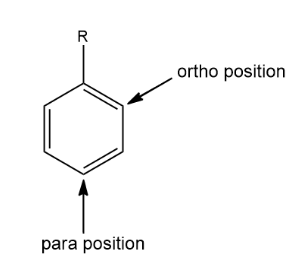
Ortho and para directing groups are the electron-donating groups. They direct the substituents to get attached at the ortho and para positions. They are also called activating groups. This is because they increase the electron density that is donating the electron to the aromatic ring thereby activating the ring and increasing the rate of the reaction. Some of the ortho-para directing groups are $ - N{H_2}, - OH, - NH - COR, - OCO - R $ etc. These are very strong activators.
When electrophilic substitution reaction takes place, and the ortho and para products are formed then among them para is considered as the major product and ortho as a minor product. The reason behind this is the steric hindrance. In the ortho product the groups are present next to each other this causes a hindrance and the group tries to attach at the para position. Hence para product predominates ortho.
Note :
There is also something known as a Meta product. This is formed when an attacking group is an electron-withdrawing group. They direct the substituent to attach at the Meta position of the aromatic ring. These groups withdraw the electrons from the benzene ring thereby decreasing the electron density of the benzene. This deactivates the ring and so these groups are also called as deactivating groups.
Complete Step By Step Answer:
First, let us understand in detail about ortho and para. These are the prefixes used to indicate the positions of a substituent on a benzene derivative or even benzene. In ortho position, the substituents occupy the positions next to each other (position $1 $ and position $2 $). In the case of para position, the substituent occupies the opposite ends (position $1 $ and position $4 $)

Ortho and para directing groups are the electron-donating groups. They direct the substituents to get attached at the ortho and para positions. They are also called activating groups. This is because they increase the electron density that is donating the electron to the aromatic ring thereby activating the ring and increasing the rate of the reaction. Some of the ortho-para directing groups are $ - N{H_2}, - OH, - NH - COR, - OCO - R $ etc. These are very strong activators.
When electrophilic substitution reaction takes place, and the ortho and para products are formed then among them para is considered as the major product and ortho as a minor product. The reason behind this is the steric hindrance. In the ortho product the groups are present next to each other this causes a hindrance and the group tries to attach at the para position. Hence para product predominates ortho.
Note :
There is also something known as a Meta product. This is formed when an attacking group is an electron-withdrawing group. They direct the substituent to attach at the Meta position of the aromatic ring. These groups withdraw the electrons from the benzene ring thereby decreasing the electron density of the benzene. This deactivates the ring and so these groups are also called as deactivating groups.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




