
Oxidation state of phosphorus in cyclotrimetaphosphoric acid is:
(A) +5
(B) +4
(C) +3
(D) None of these
Answer
591.3k+ views
Hint: The sum of the oxidation states of the constituent ions of any compound is equal to the oxidation state or charge of the entire compound. If not specified or written then the oxidation state of the compound is zero.
Complete answer:
-The oxidation state is defined as the total number of electrons that can be lost or gained by any atom to form bonds or to form any complex. If due to a reaction the oxidation state increases then it is known as oxidation and if the oxidation state decreases then it is known as reduction.
-The oxidation state or charge of the entire compound as a whole is equal to the sum of the oxidation states of its constituent ions.
-First of all we will see the molecular formula and structure of cyclotrimetaphosphoric acid. It has a molecular formula of: ${(HP{O_3})_3}$ and its structure is shown below:
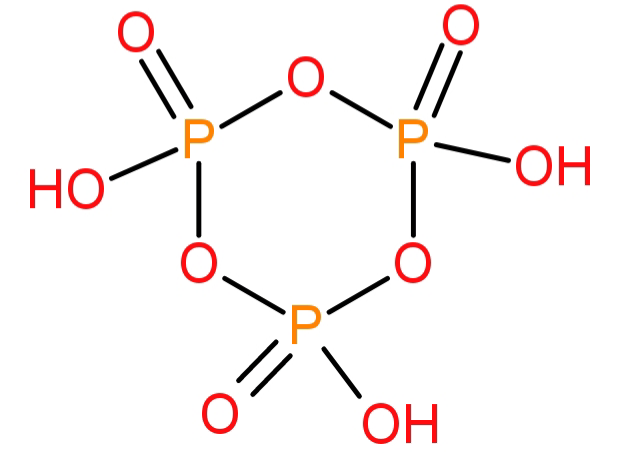
-This compound contains 15 bonds of which 12 are σ (sigma) bonds and 3 are π (pi) bonds.
-Now we will calculate the oxidation state of the given compound.
Its overall charge or oxidation state is 0, that of H is (+1) and of O is (-2). Let us assume the oxidation state of phosphorus (P) to be ‘x’.
0 = 3 [ 1 + x + 3(-2) ]
0 = 1 + x -6
0 = x – 5
x = +5
So, the oxidation state of cyclotrimetaphosphoric acid is (+5).
-Cyclotrimetaphosphoric acid belongs to the group of non-metal phosphate which are those inorganic non-metallic compounds which contain phosphate as its largest oxoanion.
So, the correct option is: (A) +5.
Note:
Cyclotrimetaphosphoric acid is an oxoacid and has acidic protons bound to the oxygen atoms. Meta phosphoric acids have a general formula of: ${(HP{O_3})_n}$ where n = 3, 4, 5.
Also phosphorus has atomic number 15 and electronic configuration of: $1{s^2}2{s^2}2{p^6}3{s^2}3{p^3}$. From this we can see that the oxidation state of phosphorus can vary from (-3) to (+5).
Complete answer:
-The oxidation state is defined as the total number of electrons that can be lost or gained by any atom to form bonds or to form any complex. If due to a reaction the oxidation state increases then it is known as oxidation and if the oxidation state decreases then it is known as reduction.
-The oxidation state or charge of the entire compound as a whole is equal to the sum of the oxidation states of its constituent ions.
-First of all we will see the molecular formula and structure of cyclotrimetaphosphoric acid. It has a molecular formula of: ${(HP{O_3})_3}$ and its structure is shown below:

-This compound contains 15 bonds of which 12 are σ (sigma) bonds and 3 are π (pi) bonds.
-Now we will calculate the oxidation state of the given compound.
Its overall charge or oxidation state is 0, that of H is (+1) and of O is (-2). Let us assume the oxidation state of phosphorus (P) to be ‘x’.
0 = 3 [ 1 + x + 3(-2) ]
0 = 1 + x -6
0 = x – 5
x = +5
So, the oxidation state of cyclotrimetaphosphoric acid is (+5).
-Cyclotrimetaphosphoric acid belongs to the group of non-metal phosphate which are those inorganic non-metallic compounds which contain phosphate as its largest oxoanion.
So, the correct option is: (A) +5.
Note:
Cyclotrimetaphosphoric acid is an oxoacid and has acidic protons bound to the oxygen atoms. Meta phosphoric acids have a general formula of: ${(HP{O_3})_n}$ where n = 3, 4, 5.
Also phosphorus has atomic number 15 and electronic configuration of: $1{s^2}2{s^2}2{p^6}3{s^2}3{p^3}$. From this we can see that the oxidation state of phosphorus can vary from (-3) to (+5).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




