
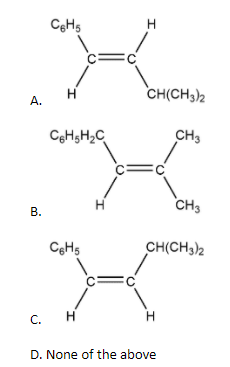
Out of the following the alkenes that exhibit optical isomerism is:

Answer
586.5k+ views
Hint: Isomerism is defined as the phenomenon in which more than one compounds have the same chemical formula but different chemical structures. Chemical compounds that have similar chemical formulae but different properties and have different arrangement of atoms in the molecule are known by isomers.
Complete Step by step solution: Primary we can categorize isomerism into two parts: Structural Isomerism and Stereoisomerism. Stereoisomerism is the type of isomerism which is present in those compounds which have the same chemical formula but different orientations of the atoms in three-dimensional space. Stereoisomers are those compounds which exhibit the property of stereoisomerism. Stereoisomerism can be further categorized into two types: geometrical isomerism and optical isomerism. Optical isomerism occurs mainly in those substances which have the same molecular and structural formula but cannot be superimposed on each other. In simple manners they are mirror images of each other. We can also differentiate them on their effect on the rotation of polarized light. To determine whether the compound is optically active or not we have to first see whether the carbon is chiral in nature or not i.e. it is attached to four different groups or not. Now consider the options in the question A, B and C no one has chiral carbon so we can say these are not optically active.
So the option D is correct in this case.
Note: Chirality and achirality of molecules can be explained on the basis of the plane of symmetry. If all the attached groups to the central carbon atom are different then there is no plane of symmetry. Such types of molecules are known as chiral molecules and if all the groups attached to the central carbon atom are not different then there is a plane of symmetry and these types of molecules are known as achiral molecules. Only molecules having a chiral center will show the property of optical isomerism.
Complete Step by step solution: Primary we can categorize isomerism into two parts: Structural Isomerism and Stereoisomerism. Stereoisomerism is the type of isomerism which is present in those compounds which have the same chemical formula but different orientations of the atoms in three-dimensional space. Stereoisomers are those compounds which exhibit the property of stereoisomerism. Stereoisomerism can be further categorized into two types: geometrical isomerism and optical isomerism. Optical isomerism occurs mainly in those substances which have the same molecular and structural formula but cannot be superimposed on each other. In simple manners they are mirror images of each other. We can also differentiate them on their effect on the rotation of polarized light. To determine whether the compound is optically active or not we have to first see whether the carbon is chiral in nature or not i.e. it is attached to four different groups or not. Now consider the options in the question A, B and C no one has chiral carbon so we can say these are not optically active.
So the option D is correct in this case.
Note: Chirality and achirality of molecules can be explained on the basis of the plane of symmetry. If all the attached groups to the central carbon atom are different then there is no plane of symmetry. Such types of molecules are known as chiral molecules and if all the groups attached to the central carbon atom are not different then there is a plane of symmetry and these types of molecules are known as achiral molecules. Only molecules having a chiral center will show the property of optical isomerism.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




