
Ortho-and para-hydrogen have
(A) Identical chemical properties but different physical properties.
(B) Identical physical and chemical properties.
(C) Identical physical properties but different chemical properties.
(D) Different physical and chemical properties.
Answer
560.4k+ views
Hint Ortho and Para hydrogen differ in the spin of their nuclei. In Ortho hydrogen molecules the spins of both the nuclei are in the same direction whereas in Para hydrogen the spins of both the nuclei are in the opposite direction. They differ in their physical properties but have similar chemical properties.
Complete Step By Step Solution: The two hydrogen atoms of hydrogen molecule in Ortho hydrogen have spin equal to one because both the hydrogen atoms have parallel nuclear spins. In case of Para hydrogen the spin of the hydrogen atoms are opposite to each other due to which they have overall spin equal to zero.
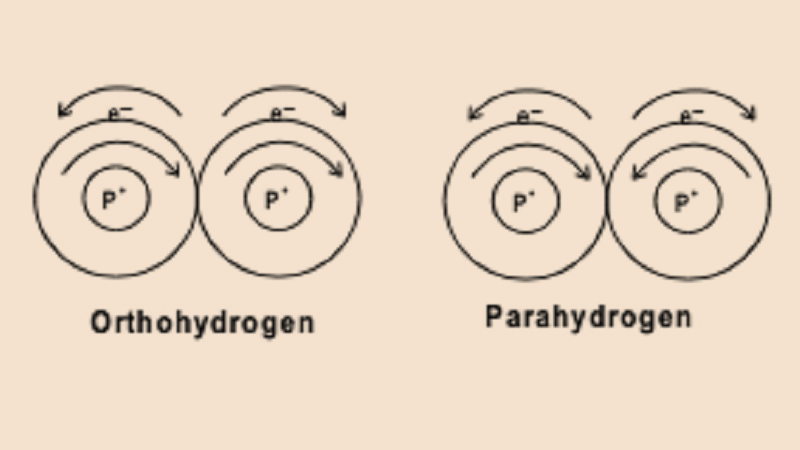
Ortho and Para hydrogen resemble each other in their chemical properties but they do have different physical properties like thermal conductivity, boiling point due to difference in their overall spins.
So, the correct choice is (A).
Additional Information:
Ortho and Para hydrogen are basically the division of hydrogen on the basis of the spin of their nuclei. Same spin of two hydrogen atoms results in Ortho hydrogen and opposite spin results in Para hydrogen. At $0{}^\circ \text{K}$, hydrogen contains mainly Para-hydrogen which is more stable than Ortho hydrogen. At room temperature the ratio of Para hydrogen to Ortho hydrogen is $1:3$. This ratio is not exceeded even if we talk about high temperatures.
It is possible to get Para hydrogen by cooling hydrogen gas to a very low temperature but the sample containing more than $75%$ of Ortho hydrogen cannot be obtained. Ordinary di-hydrogen is an equilibrium mixture of both Para and Ortho hydrogen.
Note: Ortho and Para hydrogen are spin isomers of molecular hydrogen. Ortho hydrogen is in a high energy state as compared to Para hydrogen. Ortho hydrogen is known as triplet state and Para hydrogen is known as singlet state. The two forms of molecular hydrogen were first proposed by Werner Heisenberg and Friedrich Hund in 1927. Para hydrogen can be converted into Ortho hydrogen by using catalysts like platinum or iron, by mixing the paramagnetic molecules or by heating the sample to ${{800}^{0}}\text{C}$or more.
Complete Step By Step Solution: The two hydrogen atoms of hydrogen molecule in Ortho hydrogen have spin equal to one because both the hydrogen atoms have parallel nuclear spins. In case of Para hydrogen the spin of the hydrogen atoms are opposite to each other due to which they have overall spin equal to zero.

Ortho and Para hydrogen resemble each other in their chemical properties but they do have different physical properties like thermal conductivity, boiling point due to difference in their overall spins.
So, the correct choice is (A).
Additional Information:
Ortho and Para hydrogen are basically the division of hydrogen on the basis of the spin of their nuclei. Same spin of two hydrogen atoms results in Ortho hydrogen and opposite spin results in Para hydrogen. At $0{}^\circ \text{K}$, hydrogen contains mainly Para-hydrogen which is more stable than Ortho hydrogen. At room temperature the ratio of Para hydrogen to Ortho hydrogen is $1:3$. This ratio is not exceeded even if we talk about high temperatures.
It is possible to get Para hydrogen by cooling hydrogen gas to a very low temperature but the sample containing more than $75%$ of Ortho hydrogen cannot be obtained. Ordinary di-hydrogen is an equilibrium mixture of both Para and Ortho hydrogen.
Note: Ortho and Para hydrogen are spin isomers of molecular hydrogen. Ortho hydrogen is in a high energy state as compared to Para hydrogen. Ortho hydrogen is known as triplet state and Para hydrogen is known as singlet state. The two forms of molecular hydrogen were first proposed by Werner Heisenberg and Friedrich Hund in 1927. Para hydrogen can be converted into Ortho hydrogen by using catalysts like platinum or iron, by mixing the paramagnetic molecules or by heating the sample to ${{800}^{0}}\text{C}$or more.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




