
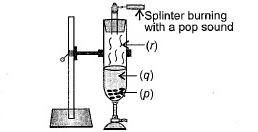
Observe the given figure carefully and identify the substances marked as (p), (q) and (r).
A. (p) - zinc, (q) - water, (r) - carbon dioxide
B. (p) -Magnesium, (q) -hydrochloric acid, (r) - oxygen
C. p) -Magnesium, (q) - water, (r) - carbon dioxide
D. p) - zinc, (q) - Hydrochloric acid, (r) - Hydrogen

Answer
589.5k+ views
Hint: The first element of periodic when brought near to the burning splinter makes the popping sound. When the gas reacts with oxygen then only it will make a pop sound. Whereas those gases who cannot react with oxygen will make a pop sound.
Complete step by step answer:
- In the given question we have to identify the three molecules or atoms that are p, q and r.
- Now, as we know that the gas which is released after the completion of reaction makes the pop sound.
- So, the gas must be hydrogen because when a burning splinter is brought near to the hydrogen gas it reacts with oxygen and makes a pop sound.
- So, according to it (r) will be hydrogen due to which option D will be the correct answer in the given question.
- Now, to prove it, we see the reaction between zinc and hydrochloric acid because according to the diagram (p) and (q) are dissolved with each other to react. So, the balanced reaction will be:
\[\text{Zn + 2HCl }\to \text{ ZnC}{{\text{l}}_{2}}\text{ + }{{\text{H}}_{2}}\]
- So, as we can see that zinc and hydrochloric acid react to the released hydrogen gas which makes the pop sound. So, the correct answer is “Option D”.
Note: The gases like carbon dioxide, oxygen don't produce a pop sound when a burning splinter is brought near to them. When magnesium reacts with water it yields hydrogen gas and not carbon dioxide. Also, when magnesium reacts with $\text{HCl}$ it yields ${{\text{H}}_{2}}$ and not ${{\text{O}}_{2}}$ and when zinc reacts with ${{\text{H}}_{2}}\text{O}$ it yields $\text{Zn(OH}{{\text{)}}_{2}}$ & ${{\text{H}}_{2}}$ and not $\text{C}{{\text{O}}_{2}}$.
Complete step by step answer:
- In the given question we have to identify the three molecules or atoms that are p, q and r.
- Now, as we know that the gas which is released after the completion of reaction makes the pop sound.
- So, the gas must be hydrogen because when a burning splinter is brought near to the hydrogen gas it reacts with oxygen and makes a pop sound.
- So, according to it (r) will be hydrogen due to which option D will be the correct answer in the given question.
- Now, to prove it, we see the reaction between zinc and hydrochloric acid because according to the diagram (p) and (q) are dissolved with each other to react. So, the balanced reaction will be:
\[\text{Zn + 2HCl }\to \text{ ZnC}{{\text{l}}_{2}}\text{ + }{{\text{H}}_{2}}\]
- So, as we can see that zinc and hydrochloric acid react to the released hydrogen gas which makes the pop sound. So, the correct answer is “Option D”.
Note: The gases like carbon dioxide, oxygen don't produce a pop sound when a burning splinter is brought near to them. When magnesium reacts with water it yields hydrogen gas and not carbon dioxide. Also, when magnesium reacts with $\text{HCl}$ it yields ${{\text{H}}_{2}}$ and not ${{\text{O}}_{2}}$ and when zinc reacts with ${{\text{H}}_{2}}\text{O}$ it yields $\text{Zn(OH}{{\text{)}}_{2}}$ & ${{\text{H}}_{2}}$ and not $\text{C}{{\text{O}}_{2}}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




