
What is the O – S – O bond angle in a $S{{O}_{3}}$molecule?
Answer
524.1k+ views
Hint: Bond angle is the angle between the adjacent atoms in the molecule. The bond angle in any molecule varies with the presence of the bond pair of electrons and the lone pair of electrons according to VSEPR theory. The $S{{O}_{3}}$ molecule is called sulfur trioxide whose geometry is trigonal planar.
Complete answer:
The shapes of molecules tell us the bond angles between them. These shapes are identified using VSEPR theory that stands for valence shell electron pair repulsion theory. This theory suggests that a shape of a molecule is dependent on the valence pair of electrons in the atoms of that molecule. These valence electrons are distributed in the form of bond pair and lone pairs. The interactions of these pairs are in the order, lone pair – lone pair > lone pair – bond pair > bond pair – bond pair. This states that lone pair lone pair interaction is more due to which the shape of the molecule distorts and the bond angle changes.
We have been given a $S{{O}_{3}}$ molecule that has sulfur and oxygen. The total valence electrons in sulfur and oxygen are 6, so the total valence electrons on the molecule will be $4\times 6=24$ valence electrons. These electrons are distributed in such a way that they form 3 electron pairs. Each pair of electrons is distributed in a way that it completes its octet, so there is no lone pair of electrons. Therefore due to 3 oxygen atoms, the shape of the molecule becomes trigonal planar. The bond angle in trigonal planar molecule is $120{}^\circ $, as the following structure suggests:
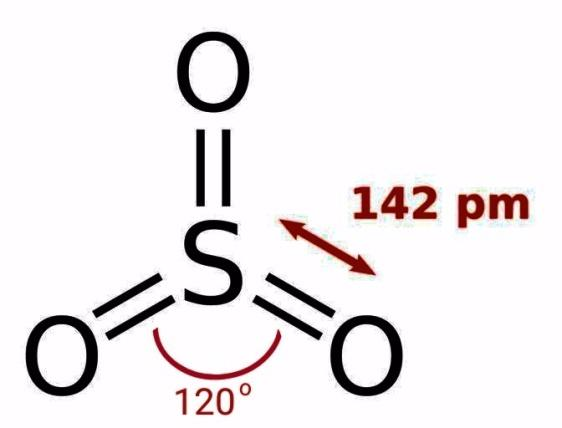
Hence, the bond angle of O – S – O bond in a $S{{O}_{3}}$ molecule is $120{}^\circ $.
Note:
$S{{O}_{3}}$ molecule is an example where the central atom is surrounded by only bond pairs and has a regular geometry. There are various molecules that are surrounded by both lone pairs and bond pairs explained in the VSEPR theory. In VSEPR theory the multiple bond like that in carbon and oxygen, $C=O$ is considered as a single electron pair, so 3 bond pairs in $S{{O}_{3}}$ molecule.
Complete answer:
The shapes of molecules tell us the bond angles between them. These shapes are identified using VSEPR theory that stands for valence shell electron pair repulsion theory. This theory suggests that a shape of a molecule is dependent on the valence pair of electrons in the atoms of that molecule. These valence electrons are distributed in the form of bond pair and lone pairs. The interactions of these pairs are in the order, lone pair – lone pair > lone pair – bond pair > bond pair – bond pair. This states that lone pair lone pair interaction is more due to which the shape of the molecule distorts and the bond angle changes.
We have been given a $S{{O}_{3}}$ molecule that has sulfur and oxygen. The total valence electrons in sulfur and oxygen are 6, so the total valence electrons on the molecule will be $4\times 6=24$ valence electrons. These electrons are distributed in such a way that they form 3 electron pairs. Each pair of electrons is distributed in a way that it completes its octet, so there is no lone pair of electrons. Therefore due to 3 oxygen atoms, the shape of the molecule becomes trigonal planar. The bond angle in trigonal planar molecule is $120{}^\circ $, as the following structure suggests:

Hence, the bond angle of O – S – O bond in a $S{{O}_{3}}$ molecule is $120{}^\circ $.
Note:
$S{{O}_{3}}$ molecule is an example where the central atom is surrounded by only bond pairs and has a regular geometry. There are various molecules that are surrounded by both lone pairs and bond pairs explained in the VSEPR theory. In VSEPR theory the multiple bond like that in carbon and oxygen, $C=O$ is considered as a single electron pair, so 3 bond pairs in $S{{O}_{3}}$ molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




