
Number of identical $Cr - O$ bonds in dichromate ion $C{r_2}{O_7}^{2 - }$ is :
(A) $4$
(B) $6$
(C) $7$
(D) $8$
Answer
567k+ views
Hint:In order to answer this question, you must recall the concept of chemical bonding and structures of ions and you must keep in mind the points of resonance and oxidation state also then only you will reach the correct answer. Firstly, try to draw the structure of the compound by taking care of the oxidation states of each element. And then observe the number of equivalent $Cr - O$ bonds by using the concept of resonance.
Complete step-by-step answer:Step 1: In this step we will figure out the number of $Cr - O$ bonds present in the structure of $C{r_2}{O_7}^{2 - }$ .
Draw the structure or refer to the figure given below, and you will observe that there are 6 $Cr - O$ present in that:
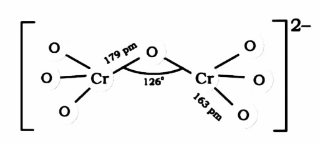
Step 2: In this step, by using the concept of resonance we will determine the equivalent $Cr - O$ bonds that are present.
Clearly, there are 6 terminal $Cr - O$ bonds. All these bonds are equivalent due to resonance.
Step 3: We know that the dichromate ions $C{r_2}{O_7}^{2 - }$ are oxoanions of chromium in the oxidation state +6.
Hence, we can conclude that there are $6$ equivalent $Cr - O$ bonds are present.
Therefore, the correct answer is option B.
Note: Dichromate is a divalent inorganic anion obtained by removal of both protons from dichromic acid. It is a chromium oxoanion and a divalent inorganic anion. It is a conjugate base of a hydrogen dichromate. Resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single Lewis formula. A molecule or ion with such delocalized electrons is represented by several resonance structures.
Complete step-by-step answer:Step 1: In this step we will figure out the number of $Cr - O$ bonds present in the structure of $C{r_2}{O_7}^{2 - }$ .
Draw the structure or refer to the figure given below, and you will observe that there are 6 $Cr - O$ present in that:

Step 2: In this step, by using the concept of resonance we will determine the equivalent $Cr - O$ bonds that are present.
Clearly, there are 6 terminal $Cr - O$ bonds. All these bonds are equivalent due to resonance.
Step 3: We know that the dichromate ions $C{r_2}{O_7}^{2 - }$ are oxoanions of chromium in the oxidation state +6.
Hence, we can conclude that there are $6$ equivalent $Cr - O$ bonds are present.
Therefore, the correct answer is option B.
Note: Dichromate is a divalent inorganic anion obtained by removal of both protons from dichromic acid. It is a chromium oxoanion and a divalent inorganic anion. It is a conjugate base of a hydrogen dichromate. Resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single Lewis formula. A molecule or ion with such delocalized electrons is represented by several resonance structures.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




