
What is the nomenclature of ${{\left[ \text{CoC}{{\text{l}}_{2}}{{\left( \text{N}{{\text{H}}_{3}} \right)}_{4}} \right]}^{+}}$.
Answer
585.3k+ views
Hint: First understand the compound structure and functional groups attached to it; by it we can determine the IUPAC name. To name any compound, there are certain sets of rules to be followed to write IUPAC names.
Complete step by step answer:
- First find the oxidation state of central metal atom which is $\text{Co}$ in ${{\left[ \text{CoC}{{\text{l}}_{2}}{{\left( \text{N}{{\text{H}}_{3}} \right)}_{4}} \right]}^{+}}$. The compound has six ligands, four ammonia and two chlorine ligands.
Let the oxidation state of $\text{Co}$ be x.
Hence, the oxidation will be$\left[ \left( \text{x} \right)+\left( -1\times 2 \right)+\left( 0\times 4 \right) \right]=+1$; so x equals to +3.
The oxidation state of $\text{Co}$ is $+3$.
The oxidation state while writing IUPAC name is written in roman numerals inside brackets.
- Now, write the names of the ligands attached.
If a central metal atom is present inside the parenthesis, then the name of the central metal atom is written like its original name; the name of cobalt will be cobalt only.
The number of chlorine atoms are 2, so the name will be ‘dichloro’. The number of ammonia molecules are 4, so the name of the ligand will be ‘tetraammine’.
The IUPAC name is written in alphabetical order.
The IUPAC name of ${{\left[ \text{CoC}{{\text{l}}_{2}}{{\left( \text{N}{{\text{H}}_{3}} \right)}_{4}} \right]}^{+}}$ will be tetraamminedichloro cobalt $\left( \text{III} \right)$.
Additional Information: There are two types of isomers of ${{\left[ \text{CoC}{{\text{l}}_{2}}{{\left( \text{N}{{\text{H}}_{3}} \right)}_{4}} \right]}^{+}}$ are cis isomers and trans isomers. These structures of cis-tetraamminedichloro cobalt $\left( \text{III} \right)$ and trans-tetraamminedichloro cobalt $\left( \text{III} \right)$ are:
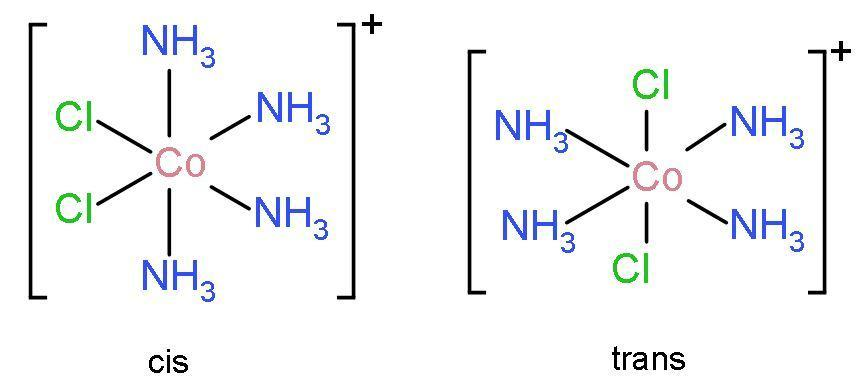
In trans-tetraamminedichloro cobalt $\left( \text{III} \right)$, chlorine ligands are present adjacent to each other.
In cis- tetraamminedichloro cobalt $\left( \text{III} \right)$, chlorine ligands are present on opposite side to each other.
Note: The name of the compound is written with substituents in alphabetical order followed by its base name. Between numbers, commas are used and dashes are used between letters and numbers.
Complete step by step answer:
- First find the oxidation state of central metal atom which is $\text{Co}$ in ${{\left[ \text{CoC}{{\text{l}}_{2}}{{\left( \text{N}{{\text{H}}_{3}} \right)}_{4}} \right]}^{+}}$. The compound has six ligands, four ammonia and two chlorine ligands.
Let the oxidation state of $\text{Co}$ be x.
Hence, the oxidation will be$\left[ \left( \text{x} \right)+\left( -1\times 2 \right)+\left( 0\times 4 \right) \right]=+1$; so x equals to +3.
The oxidation state of $\text{Co}$ is $+3$.
The oxidation state while writing IUPAC name is written in roman numerals inside brackets.
- Now, write the names of the ligands attached.
If a central metal atom is present inside the parenthesis, then the name of the central metal atom is written like its original name; the name of cobalt will be cobalt only.
The number of chlorine atoms are 2, so the name will be ‘dichloro’. The number of ammonia molecules are 4, so the name of the ligand will be ‘tetraammine’.
The IUPAC name is written in alphabetical order.
The IUPAC name of ${{\left[ \text{CoC}{{\text{l}}_{2}}{{\left( \text{N}{{\text{H}}_{3}} \right)}_{4}} \right]}^{+}}$ will be tetraamminedichloro cobalt $\left( \text{III} \right)$.
Additional Information: There are two types of isomers of ${{\left[ \text{CoC}{{\text{l}}_{2}}{{\left( \text{N}{{\text{H}}_{3}} \right)}_{4}} \right]}^{+}}$ are cis isomers and trans isomers. These structures of cis-tetraamminedichloro cobalt $\left( \text{III} \right)$ and trans-tetraamminedichloro cobalt $\left( \text{III} \right)$ are:

In trans-tetraamminedichloro cobalt $\left( \text{III} \right)$, chlorine ligands are present adjacent to each other.
In cis- tetraamminedichloro cobalt $\left( \text{III} \right)$, chlorine ligands are present on opposite side to each other.
Note: The name of the compound is written with substituents in alphabetical order followed by its base name. Between numbers, commas are used and dashes are used between letters and numbers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




