
Neopentane and isopentane are:
(A) Allotropes
(B) Isobars
(C) Isomers
(D) Homologous
Answer
587.7k+ views
Hint: Find out the meaning of each of the terms present in the given options. Draw neopentane and isopentane and then relate with the option terms to find out the answer.
Complete answer:
- Let’s have a look at the options given in the question.
- Allotropes are different forms of the same element. Allotropes are formed due different structural arrangements of constituent atoms or molecules. Some allotropes of carbon are diamond, graphite, coke, etc.
- Isobars are elements containing the same atomic mass number but different atomic numbers. For example, ${}^{40}S$, ${}^{40}K$, ${}^{40}Ca$.
- Isomers are compounds having the same molecular formula but different structural formulae are called isomers. This property is known as isomerism. For example, ethanol and diethyl ether, isopropanol and n-propanol.
- Homologous refers to homologous series which is a series of organic compounds each having a characteristic functional group and the successive members differ from each other in molecular formula by one methylene $\left( -C{{H}_{2}} \right)$ group. For example, alkanes, alkenes, alcohols, amines, carboxylic acids, ethers, etc.
- In the question, we need to find in which of the above groups does neopentane and isopentane fit in. Let’s first draw their structures.
- Structure of Neopentane is,
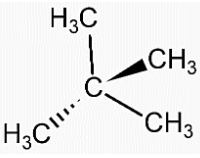
- Structure of isopentane is,
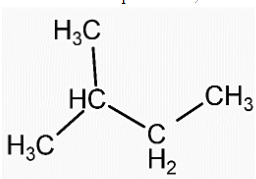
- We can see that in both the compounds the number of carbon and hydrogen atoms is the same. That means both the compounds have the same molecular formula, ${{C}_{5}}{{H}_{12}}$.
- Therefore, neopentane and isopentane are isomers of each other. Also, both the compounds are alkanes so they also are homologous.
The correct answers are option (C) and (D).
Note:
Draw the structures of compounds in such types of questions to determine in which category they belong. In this example, the easiest way to identify was from the name itself. In both the compounds pentane is there and prefix ‘iso’ and ‘neo’ are added which represent type of branching but overall, the compound has 5 carbon atoms with alkane as functional group, so they are homologous as well as isomers of each other.
Complete answer:
- Let’s have a look at the options given in the question.
- Allotropes are different forms of the same element. Allotropes are formed due different structural arrangements of constituent atoms or molecules. Some allotropes of carbon are diamond, graphite, coke, etc.
- Isobars are elements containing the same atomic mass number but different atomic numbers. For example, ${}^{40}S$, ${}^{40}K$, ${}^{40}Ca$.
- Isomers are compounds having the same molecular formula but different structural formulae are called isomers. This property is known as isomerism. For example, ethanol and diethyl ether, isopropanol and n-propanol.
- Homologous refers to homologous series which is a series of organic compounds each having a characteristic functional group and the successive members differ from each other in molecular formula by one methylene $\left( -C{{H}_{2}} \right)$ group. For example, alkanes, alkenes, alcohols, amines, carboxylic acids, ethers, etc.
- In the question, we need to find in which of the above groups does neopentane and isopentane fit in. Let’s first draw their structures.
- Structure of Neopentane is,

- Structure of isopentane is,

- We can see that in both the compounds the number of carbon and hydrogen atoms is the same. That means both the compounds have the same molecular formula, ${{C}_{5}}{{H}_{12}}$.
- Therefore, neopentane and isopentane are isomers of each other. Also, both the compounds are alkanes so they also are homologous.
The correct answers are option (C) and (D).
Note:
Draw the structures of compounds in such types of questions to determine in which category they belong. In this example, the easiest way to identify was from the name itself. In both the compounds pentane is there and prefix ‘iso’ and ‘neo’ are added which represent type of branching but overall, the compound has 5 carbon atoms with alkane as functional group, so they are homologous as well as isomers of each other.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




