
What is the molecular formula of a diamond?
Answer
534.9k+ views
Hint: Allotropy is the property of any element to exist in more than one form. Diamond, graphite, coke, etc. are called allotropes of carbon. The structure and the position of the atoms in the diamond lattice tell us the molecular formula of diamond and the nature of its bonds.
Complete answer:
Allotropes are the different forms that are possessed by an element that has different structural variations and chemical variations. The most common allotropes can be seen in carbon. Carbon possesses allotropy and has different forms like, graphite, diamond, coke, graphenes, fullerenes, etc. These forms consist of carbon as a sole atom which has different arrangement patterns in their respective crystal lattice.
Diamond is one of the allotropes of carbon that is a crystalline, solid, and homogenous form of carbon. Diamond consists of a 3 – dimensional structure of carbon network. The carbon atoms are $s{{p}^{3}}$ hybridized and form a tetrahedral geometry. Each carbon atom is linked with four other carbon atoms which are again linked with four carbons, which forms a network. These carbons are linked together with covalent bonds. The structure of diamond is:
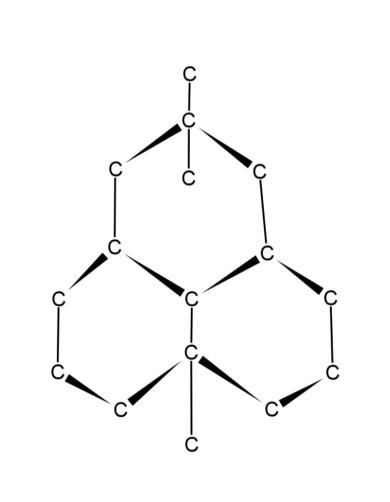
Hence, the molecular formula of diamond is C as it consists of only carbon atoms.
Note:
In diamond carbon is linked with 4 other carbons, so it has a C – 4 arrangement, while in graphite, the carbon atoms are linked with 6 other carbons, so it has a C – 6 arrangement. These are the crystalline allotropes of carbon. While coke, coal and soot are termed as amorphous allotropes of carbon.
Complete answer:
Allotropes are the different forms that are possessed by an element that has different structural variations and chemical variations. The most common allotropes can be seen in carbon. Carbon possesses allotropy and has different forms like, graphite, diamond, coke, graphenes, fullerenes, etc. These forms consist of carbon as a sole atom which has different arrangement patterns in their respective crystal lattice.
Diamond is one of the allotropes of carbon that is a crystalline, solid, and homogenous form of carbon. Diamond consists of a 3 – dimensional structure of carbon network. The carbon atoms are $s{{p}^{3}}$ hybridized and form a tetrahedral geometry. Each carbon atom is linked with four other carbon atoms which are again linked with four carbons, which forms a network. These carbons are linked together with covalent bonds. The structure of diamond is:

Hence, the molecular formula of diamond is C as it consists of only carbon atoms.
Note:
In diamond carbon is linked with 4 other carbons, so it has a C – 4 arrangement, while in graphite, the carbon atoms are linked with 6 other carbons, so it has a C – 6 arrangement. These are the crystalline allotropes of carbon. While coke, coal and soot are termed as amorphous allotropes of carbon.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




