
What is molar conductivity at infinite dilution?
Answer
509.4k+ views
Hint: We know that in an electrolytic solution, the flow of electricity is due to migration of ions when a potential difference is applied among two electrodes. The positively charged ions i.e., cations move towards negatively charged electrodes known as cathode whereas the negatively charged ions i.e., anions move towards positively charged electrodes known as anodes. The ease with which the flow of electricity takes place within a solution is called conductance and it is reciprocal of resistance.
Complete answer:
Molar conductance: It is defined as the conducting power of all the migrating ions produced by one mole of electrolyte in the aqueous solution. It is represented by the symbol ${ \wedge _m}$. It can be expressed in terms of specific conductance and concentration as follows:
${ \wedge _m} = {\text{specific conductance }} \times \dfrac{1}{c}$
$ \Rightarrow { \wedge _m} = \dfrac{\kappa }{c}$
Where, $\kappa $ is the specific conductance and c is the concentration of electrolyte.
Variation of molar conductance with dilution (Molar conductance at infinite dilution):
The molar conductance of an electrolyte generally increases with increase in dilution that means the same amount of electrolyte will have a tendency to form more number ions on dilution. But in actuality, the increase in volume of solution is much more than the number of ions generated. Therefore, in actuality it is observed that the number of ions per unit volume decreases and thus specific conductance also decreases although on progressive dilution, the value of molar conductance increases.
The variation for molar conductance for strong and weak electrolytes can be represented through a graph which is as follows:
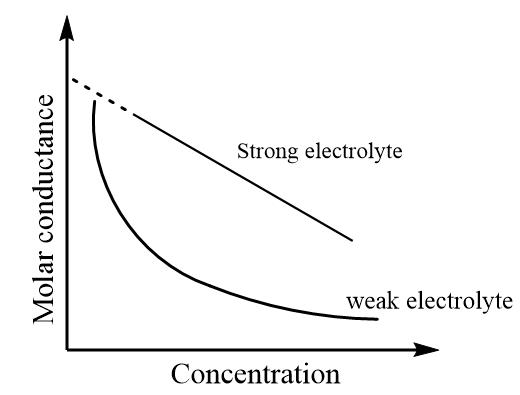
As it is observed in the graph that, for strong electrolytes there is a tendency for molar conductance to achieve a certain limiting value when the concentration of the solution approaches zero. The molar conductance at this point is termed as molar conductance at infinite dilution. It is represented by the symbol $ \wedge _m^o$.
Note:
It should be kept in mind that the graph for strong electrolytes is linear, so the value of molar conductance at infinite dilution can be calculated by extrapolation of the curve. But in case of weak electrolytes, the curve is not linear, so the value of $ \wedge _m^o$ cannot be determined by extrapolating its curve. Instead, it is calculated by an indirect method based on Kohlrausch’s law.
Complete answer:
Molar conductance: It is defined as the conducting power of all the migrating ions produced by one mole of electrolyte in the aqueous solution. It is represented by the symbol ${ \wedge _m}$. It can be expressed in terms of specific conductance and concentration as follows:
${ \wedge _m} = {\text{specific conductance }} \times \dfrac{1}{c}$
$ \Rightarrow { \wedge _m} = \dfrac{\kappa }{c}$
Where, $\kappa $ is the specific conductance and c is the concentration of electrolyte.
Variation of molar conductance with dilution (Molar conductance at infinite dilution):
The molar conductance of an electrolyte generally increases with increase in dilution that means the same amount of electrolyte will have a tendency to form more number ions on dilution. But in actuality, the increase in volume of solution is much more than the number of ions generated. Therefore, in actuality it is observed that the number of ions per unit volume decreases and thus specific conductance also decreases although on progressive dilution, the value of molar conductance increases.
The variation for molar conductance for strong and weak electrolytes can be represented through a graph which is as follows:

As it is observed in the graph that, for strong electrolytes there is a tendency for molar conductance to achieve a certain limiting value when the concentration of the solution approaches zero. The molar conductance at this point is termed as molar conductance at infinite dilution. It is represented by the symbol $ \wedge _m^o$.
Note:
It should be kept in mind that the graph for strong electrolytes is linear, so the value of molar conductance at infinite dilution can be calculated by extrapolation of the curve. But in case of weak electrolytes, the curve is not linear, so the value of $ \wedge _m^o$ cannot be determined by extrapolating its curve. Instead, it is calculated by an indirect method based on Kohlrausch’s law.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




