
How many lone pair(s) is/are present in the central atom $I$ of $I_{3}^{-}$ ion?
Answer
591.6k+ views
Hint: Think about the number of valence electrons that iodine has and how it hybridizes to form bonds. The hybridization and the valence electrons, along with the number of bonds formed will tell you how many lone pairs exist.
Complete step by step answer:
We know that the number of valence electrons in an Iodine atom is 7. Since the charge of the molecule is -1, one more electron is added to the compound. Now the total number of electrons in the compound has become 8. Out of which, two electrons are used to form covalent bonds with two other iodine atoms. Remaining number of electrons that are available is 6. That means, three pairs of electrons. Therefore, the number of lone pairs is 3. The structure of the $I_{3}^{-}$ ion is:
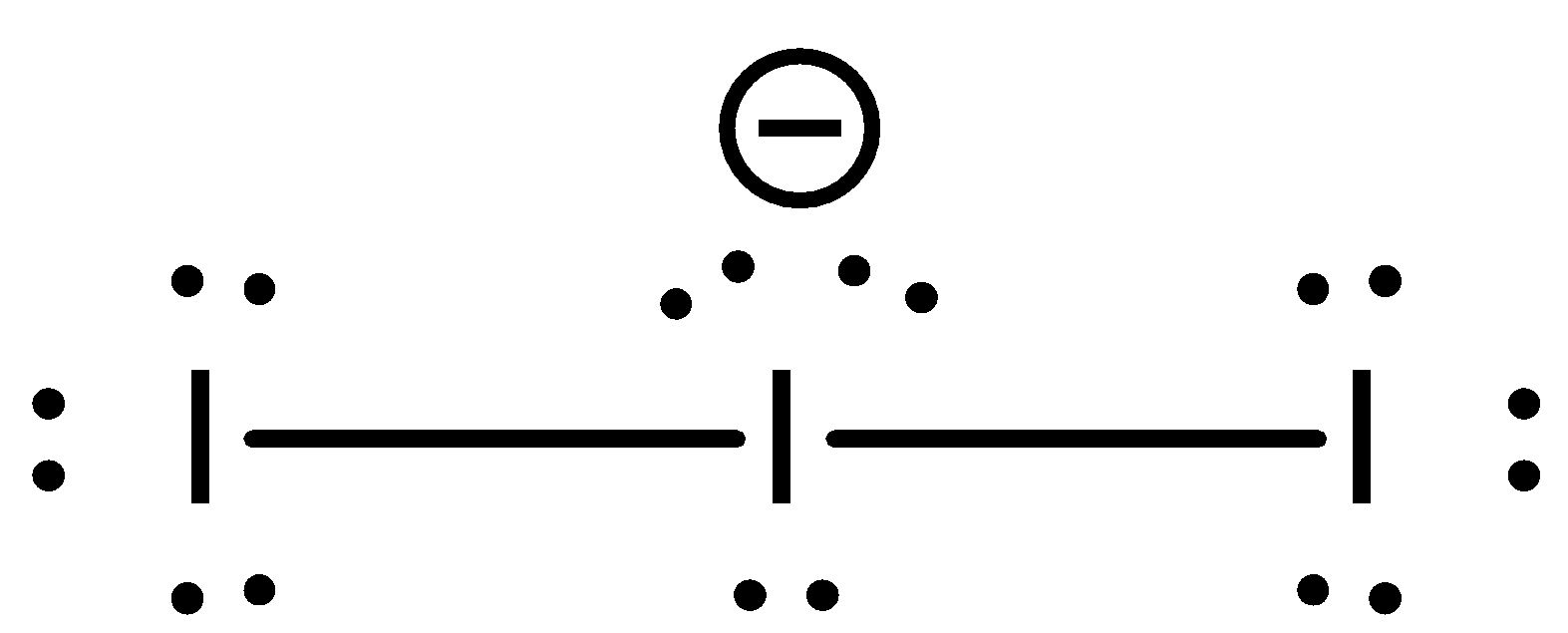
This central atom hybridizes in a trigonal bipyramidal structure. The lone pairs are present at the vertices on the triangle (equatorial) and the iodine atoms are present at the vertices of the pyramids. The hybridized structure of this atom will be:
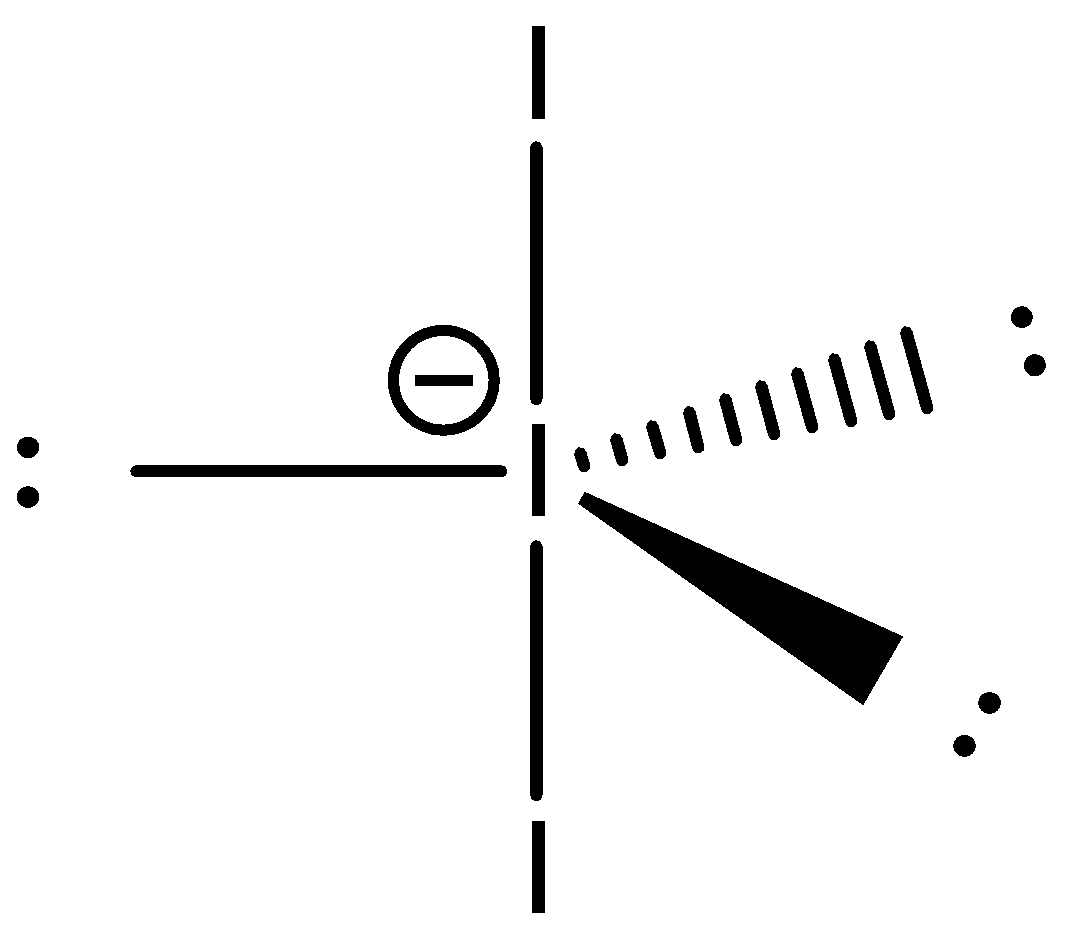
Additional Information:
- We know that the Iodine atoms in the $I_{3}^{-}$ compound are bonded through covalent bonding. Covalent bonding is the bonding in which mutual sharing of electrons take place.
- Also, the molecular geometry of $I_{3}^{-}$ is linear. While there are three Iodine atoms, one of the atoms has a negative charge which further gives three lone pairs of electrons and two bond pairs. Its steric number is five.
- Here, the name of the compound $I_{3}^{-}$ is Triiodide ion.
- Bond angle of this compound is 180 which means the shape of the molecule is linear.
- Hybridization of this compound is $s{{p}^{3}}d$ which shows the electronic geometry is trigonal bipyramidal but the shape is linear since the three equatorial positions are filled with three lone pairs of electrons.
Note: $I_{3}^{-}$ is formed by the bonding of ${{I}_{2}}$ with ${{I}^{-}}$ ion. During the combination of Iodine atoms, the central atom gains a negative charge whose value will be 1. ${{I}^{-}}$ Ion is the donor and ${{I}_{2}}$ molecule is the acceptor. Electrons are mostly accommodated in the empty d orbitals.
Complete step by step answer:
We know that the number of valence electrons in an Iodine atom is 7. Since the charge of the molecule is -1, one more electron is added to the compound. Now the total number of electrons in the compound has become 8. Out of which, two electrons are used to form covalent bonds with two other iodine atoms. Remaining number of electrons that are available is 6. That means, three pairs of electrons. Therefore, the number of lone pairs is 3. The structure of the $I_{3}^{-}$ ion is:

This central atom hybridizes in a trigonal bipyramidal structure. The lone pairs are present at the vertices on the triangle (equatorial) and the iodine atoms are present at the vertices of the pyramids. The hybridized structure of this atom will be:

Additional Information:
- We know that the Iodine atoms in the $I_{3}^{-}$ compound are bonded through covalent bonding. Covalent bonding is the bonding in which mutual sharing of electrons take place.
- Also, the molecular geometry of $I_{3}^{-}$ is linear. While there are three Iodine atoms, one of the atoms has a negative charge which further gives three lone pairs of electrons and two bond pairs. Its steric number is five.
- Here, the name of the compound $I_{3}^{-}$ is Triiodide ion.
- Bond angle of this compound is 180 which means the shape of the molecule is linear.
- Hybridization of this compound is $s{{p}^{3}}d$ which shows the electronic geometry is trigonal bipyramidal but the shape is linear since the three equatorial positions are filled with three lone pairs of electrons.
Note: $I_{3}^{-}$ is formed by the bonding of ${{I}_{2}}$ with ${{I}^{-}}$ ion. During the combination of Iodine atoms, the central atom gains a negative charge whose value will be 1. ${{I}^{-}}$ Ion is the donor and ${{I}_{2}}$ molecule is the acceptor. Electrons are mostly accommodated in the empty d orbitals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




