
Liebig’s combustion method is used for the quantitative estimation of ____________.
(A) C and H
(B) Halogens
(C) S and P
(D) N
Answer
594.9k+ views
Hint: Try to recall that the principle of Liebig’s is to strongly heat a known mass of organic compounds with excess of dry copper oxide and under these conditions, carbon dioxide and water is produced. Now, by using this you can easily find the correct option from the given ones.
Complete step by step solution:
- In Liebig’s test, a known mass of organic compound is heated strongly with excess of dry copper oxide in presence of pure oxygen and the carbon present is oxidised to carbon dioxide and hydrogen is oxidised to water. The chemical reaction of combustion can be given as under.
\[{{C}_{x}}{{H}_{y}}+(x+\dfrac{y}{4}){{O}_{2}}\to xC{{O}_{2}}+\dfrac{y}{2}{{H}_{2}}O\]
- The combustion products first pass through a U shaped tube containing anhydrous calcium chloride and then, through the caustic potash bottle. Anhydrous calcium chloride absorbs the water content and caustic potash reacts with carbon dioxide gas.
- The carbon dioxide and water formed are collected and weighted.
- The percentage of carbon and hydrogen in the compound is calculated from the mass of carbon dioxide and water produced. The apparatus arrangement for Liebig’s test is shown below.
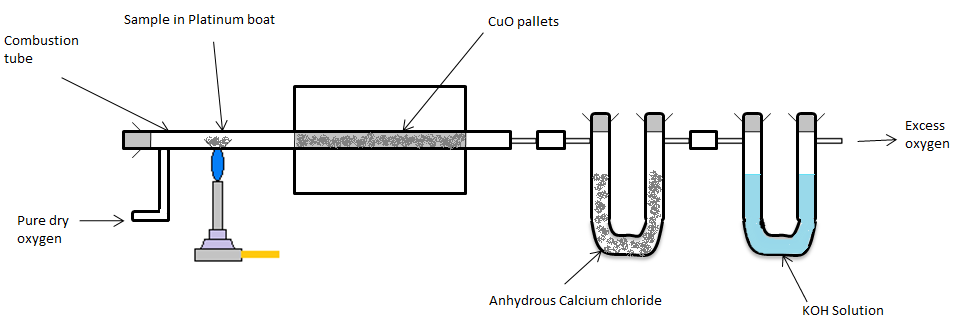
- Estimation of carbon and hydrogen in an organic compound is based on their conversion to carbon dioxide and water respectively. So, Liebig’s test is suitable for the estimation of carbon and hydrogen present in organic compounds.
Therefore, from above we can conclude that option A is the correct option to the given question.
Note: It should be remembered that under the conditions of combustion, nitrogen if present in the organic compound is also oxidised to oxides of nitrogen which are also absorbed in caustic soda bottles. In such cases, reduced copper gauze is placed near the exit end of the tube which reduces oxides of nitrogen back to nitrogen gas.
Complete step by step solution:
- In Liebig’s test, a known mass of organic compound is heated strongly with excess of dry copper oxide in presence of pure oxygen and the carbon present is oxidised to carbon dioxide and hydrogen is oxidised to water. The chemical reaction of combustion can be given as under.
\[{{C}_{x}}{{H}_{y}}+(x+\dfrac{y}{4}){{O}_{2}}\to xC{{O}_{2}}+\dfrac{y}{2}{{H}_{2}}O\]
- The combustion products first pass through a U shaped tube containing anhydrous calcium chloride and then, through the caustic potash bottle. Anhydrous calcium chloride absorbs the water content and caustic potash reacts with carbon dioxide gas.
- The carbon dioxide and water formed are collected and weighted.
- The percentage of carbon and hydrogen in the compound is calculated from the mass of carbon dioxide and water produced. The apparatus arrangement for Liebig’s test is shown below.

- Estimation of carbon and hydrogen in an organic compound is based on their conversion to carbon dioxide and water respectively. So, Liebig’s test is suitable for the estimation of carbon and hydrogen present in organic compounds.
Therefore, from above we can conclude that option A is the correct option to the given question.
Note: It should be remembered that under the conditions of combustion, nitrogen if present in the organic compound is also oxidised to oxides of nitrogen which are also absorbed in caustic soda bottles. In such cases, reduced copper gauze is placed near the exit end of the tube which reduces oxides of nitrogen back to nitrogen gas.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




