
$\left[ PdC{{l}_{2}}{{\left( PM{{e}_{3}} \right)}_{2}} \right]$ is a diamagnetic complex of Pd (II). How many unpaired electrons are present in the analogous complex of Ni (II)?
A. Zero
B. 1
C. 2
D. 3
Answer
566.7k+ views
Hint: $\left[ PdC{{l}_{2}}{{\left( PM{{e}_{3}} \right)}_{2}} \right]$ is a square planar complex and it is diamagnetic in nature. If a coordination complex has unpaired electrons then the complex is paramagnetic in nature.
Complete answer:
- We have to find that the complex $\left[ NiC{{l}_{2}}{{\left( PM{{e}_{3}} \right)}_{2}} \right]$ has unpaired electrons or not.
- First we will discuss the orbital splitting and filling of electrons in the orbitals of the complex $\left[ PdC{{l}_{2}}{{\left( PM{{e}_{3}} \right)}_{2}} \right]$ .
- The d orbital splitting of palladium metal has occurred due to the presence of ligands.
- The d-orbital splitting and the filling of electrons in the new energy levels is different, and not following the Hund's rule and the Aufbau principle.
- The energy splitting of the d-orbitals of the palladium is as follows.
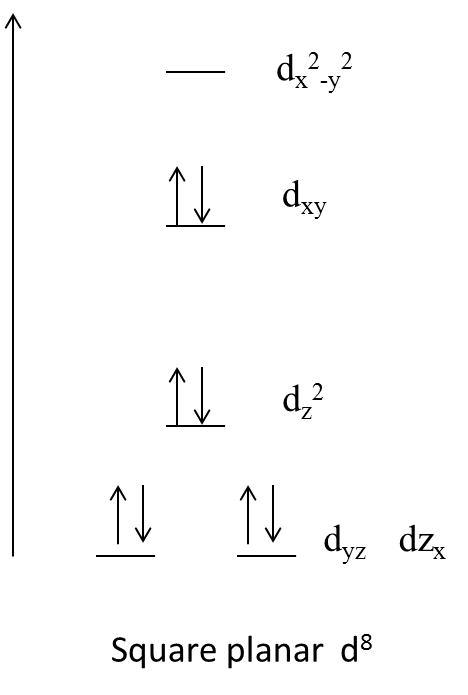
- This is why $\left[ PdC{{l}_{2}}{{\left( PM{{e}_{3}} \right)}_{2}} \right]$ complex does not contain unpaired electrons and diamagnetic in nature.
- Coming to the analogues of Nickel $\left[ NiC{{l}_{2}}{{\left( PM{{e}_{3}} \right)}_{2}} \right]$
- It has the following pattern of splitting of d-orbitals and filling of electrons in the d-orbitals.

- From the above image we can say that there are two unpaired electrons in the d-orbitals of the nickel and the complex is paramagnetic in nature.
- Therefore the number of unpaired electrons present in the analogous complex of Ni (II) is 2.
- So, the correct option is C.
Note: Both the complexes have the same structure square planar but the arrangement of electrons are different in the d-orbitals. Due to this reason $\left[ PdC{{l}_{2}}{{\left( PM{{e}_{3}} \right)}_{2}} \right]$ is diamagnetic and $\left[ NiC{{l}_{2}}{{\left( PM{{e}_{3}} \right)}_{2}} \right]$ is paramagnetic in nature.
Complete answer:
- We have to find that the complex $\left[ NiC{{l}_{2}}{{\left( PM{{e}_{3}} \right)}_{2}} \right]$ has unpaired electrons or not.
- First we will discuss the orbital splitting and filling of electrons in the orbitals of the complex $\left[ PdC{{l}_{2}}{{\left( PM{{e}_{3}} \right)}_{2}} \right]$ .
- The d orbital splitting of palladium metal has occurred due to the presence of ligands.
- The d-orbital splitting and the filling of electrons in the new energy levels is different, and not following the Hund's rule and the Aufbau principle.
- The energy splitting of the d-orbitals of the palladium is as follows.

- This is why $\left[ PdC{{l}_{2}}{{\left( PM{{e}_{3}} \right)}_{2}} \right]$ complex does not contain unpaired electrons and diamagnetic in nature.
- Coming to the analogues of Nickel $\left[ NiC{{l}_{2}}{{\left( PM{{e}_{3}} \right)}_{2}} \right]$
- It has the following pattern of splitting of d-orbitals and filling of electrons in the d-orbitals.

- From the above image we can say that there are two unpaired electrons in the d-orbitals of the nickel and the complex is paramagnetic in nature.
- Therefore the number of unpaired electrons present in the analogous complex of Ni (II) is 2.
- So, the correct option is C.
Note: Both the complexes have the same structure square planar but the arrangement of electrons are different in the d-orbitals. Due to this reason $\left[ PdC{{l}_{2}}{{\left( PM{{e}_{3}} \right)}_{2}} \right]$ is diamagnetic and $\left[ NiC{{l}_{2}}{{\left( PM{{e}_{3}} \right)}_{2}} \right]$ is paramagnetic in nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




