
IUPAC name of isobutyraldehyde is:
A) Butanal
B) 2-methylpropanal
C) Ethyl ethanol
D) Methyl butanal
Answer
566.7k+ views
Hint: The IUPAC name for aldehydes end with –al.
The number of carbons should be noted. As the term “buty” is present, the compound should have 4 carbons.
Complete Solution :
In the question they have asked for the IUPAC name of isobutyraldehyde, there are few rules set by IUPAC.
Let’s see the rules for the nomenclature:
-First identify the number of carbons in the chain .Depending on the number of C the chain is named as methane, ethane, propane etc.
-If functional groups are present in the compound then it may be given as prefix or suffix.
-If the functional group is present and the name is given as a suffix, then the name is modified by eliminating the –e from the main chain name and will give the name of the functional group.
Example – for aldehyde its al, for ketone it is –one
-For a chain containing 2 carbon, the chain name is ethane and if aldehyde is present as the functional group, then the name will be propanal.
-If in the carbon chain, there is a double bond then the name will end with –ene and if triple bond is present then the name will end like –yne.
So now let’s decode the common name given and write the answer:
Isobutyraldehyde- from the name we can say that the functional group present here is aldehyde, the number of carbons present is four. And prefix -iso represents which type of isomer is it.
So by keeping the IUPAC rules in mind, For aldehyde group we give suffix as al
4C carbons present in the formulae but the C chain taken is propane.
The longest chain should be taken in that case the carbon with long chain along with the functional group, so here methyl group is the coming as the substituent in the second carbon.
For more clarity, the structure is given below:
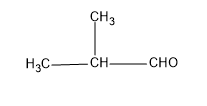
So the final answer, the IUPAC name, will be 2-methyl propanal.
So, the correct answer is “Option B”.
Note: Isobutyraldehyde come under the class of propanals i.e. isobutyraldehyde is propanal with ‘methyl group in the second carbon of the propane main chain, some may write as butanal since there are four C atom in the molecule.
The number of carbons should be noted. As the term “buty” is present, the compound should have 4 carbons.
Complete Solution :
In the question they have asked for the IUPAC name of isobutyraldehyde, there are few rules set by IUPAC.
Let’s see the rules for the nomenclature:
-First identify the number of carbons in the chain .Depending on the number of C the chain is named as methane, ethane, propane etc.
-If functional groups are present in the compound then it may be given as prefix or suffix.
-If the functional group is present and the name is given as a suffix, then the name is modified by eliminating the –e from the main chain name and will give the name of the functional group.
Example – for aldehyde its al, for ketone it is –one
-For a chain containing 2 carbon, the chain name is ethane and if aldehyde is present as the functional group, then the name will be propanal.
-If in the carbon chain, there is a double bond then the name will end with –ene and if triple bond is present then the name will end like –yne.
So now let’s decode the common name given and write the answer:
Isobutyraldehyde- from the name we can say that the functional group present here is aldehyde, the number of carbons present is four. And prefix -iso represents which type of isomer is it.
So by keeping the IUPAC rules in mind, For aldehyde group we give suffix as al
4C carbons present in the formulae but the C chain taken is propane.
The longest chain should be taken in that case the carbon with long chain along with the functional group, so here methyl group is the coming as the substituent in the second carbon.
For more clarity, the structure is given below:

So the final answer, the IUPAC name, will be 2-methyl propanal.
So, the correct answer is “Option B”.
Note: Isobutyraldehyde come under the class of propanals i.e. isobutyraldehyde is propanal with ‘methyl group in the second carbon of the propane main chain, some may write as butanal since there are four C atom in the molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




