
Is Nitrogen a flammable gas?
Answer
505.2k+ views
Hint: Flammable substances are those substances which catch fire on a small ignition. Any substance or gas becomes flammable when the gas is unstable in nature. The substance which is unstable in nature has high entropy and thus possesses high energy. Therefore high energy means there is a large chance of explosion or we can find that its combustion is easy.
Complete answer: In our environments gases are the most flammable substance. This is because gases have high entropy and thus the particles of gases are in random and thermal motion continuously. In some gases a small ignition is enough to cause an explosion. But nitrogen is a non-flammable gas which means it does not catch fire. Most gases catch fire because of their un- stability. Due to which the electrons of such gases are in the highest energy state and thus catch fire on a small ignition. Whereas if we see the electronic configuration of nitrogen it is $1{s^2}{\text{ 2}}{{\text{s}}^2}{\text{ 2}}{{\text{p}}^3}$ .
We can observe that it is a stable configuration as $p$ orbital is half filled. When two Nitrogen atoms combine they form a ${N_2}$ molecule. It can be represented as:
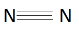
Here we can see that a triple bond is present in between the two nitrogen atoms. One of the bonds is a coordinate bond while others are sigma and pi bonds. Thus the molecule has high enthalpy for combustion. Hence nitrogen is not a flammable gas.
Note:
Nitrogen gas is non reactive in nature. It reacts only at high temperatures. Also it is a nontoxic gas and also it is colourless because all the electrons are in the ground state. Therefore it does not support the combustion of substances.
Complete answer: In our environments gases are the most flammable substance. This is because gases have high entropy and thus the particles of gases are in random and thermal motion continuously. In some gases a small ignition is enough to cause an explosion. But nitrogen is a non-flammable gas which means it does not catch fire. Most gases catch fire because of their un- stability. Due to which the electrons of such gases are in the highest energy state and thus catch fire on a small ignition. Whereas if we see the electronic configuration of nitrogen it is $1{s^2}{\text{ 2}}{{\text{s}}^2}{\text{ 2}}{{\text{p}}^3}$ .
We can observe that it is a stable configuration as $p$ orbital is half filled. When two Nitrogen atoms combine they form a ${N_2}$ molecule. It can be represented as:

Here we can see that a triple bond is present in between the two nitrogen atoms. One of the bonds is a coordinate bond while others are sigma and pi bonds. Thus the molecule has high enthalpy for combustion. Hence nitrogen is not a flammable gas.
Note:
Nitrogen gas is non reactive in nature. It reacts only at high temperatures. Also it is a nontoxic gas and also it is colourless because all the electrons are in the ground state. Therefore it does not support the combustion of substances.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




