
Is Ferrocene soluble in water?
Answer
535.8k+ views
Hint: Ferrocene is a best example for organometallic compounds. In ferrocene one iron atom is sandwiched between two cyclopentadiene rings. Means ferrocene contains one iron atom and two cyclopentadiene rings. This is the reason ferrocene is called an organometallic compound.
Complete step-by-step answer:- In the question it is asked if ferrocene is soluble in water.
- We should know the structure of the ferrocene before we are going to discuss the solubility of the ferrocene.
- The structure of the ferrocene is as follows:
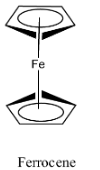
- In the structure we can see that there are two cyclopentadiene rings and one iron atom which is sandwiched in between the two cyclopentadiene rings.
- Therefore because of the presence of the cyclopentadienyl rings (organic molecules) ferrocene is not going to be soluble in water.
- But ferrocene is soluble in organic solvents like benzene and highly acidic compounds like nitric acid and concentrated sulphuric acid and etc.
- Ferrocene is a crystalline solid and possesses yellow-orange color.
- The oxidation state of iron in ferrocene is ‘+2’ .
- Here the cyclopentadiene rings donates the electrons to the iron like ligands and iron forms organometallic complexes easily with the two cyclopentadiene rings.
Note: Ferrocene undergoes substitution reactions at cyclopentadiene rings and easily produces substituted products. Ferrocene has eclipsed conformation and it is stable in nature. The derivatives of ferrocene have a lot of applications in material science.
Complete step-by-step answer:- In the question it is asked if ferrocene is soluble in water.
- We should know the structure of the ferrocene before we are going to discuss the solubility of the ferrocene.
- The structure of the ferrocene is as follows:

- In the structure we can see that there are two cyclopentadiene rings and one iron atom which is sandwiched in between the two cyclopentadiene rings.
- Therefore because of the presence of the cyclopentadienyl rings (organic molecules) ferrocene is not going to be soluble in water.
- But ferrocene is soluble in organic solvents like benzene and highly acidic compounds like nitric acid and concentrated sulphuric acid and etc.
- Ferrocene is a crystalline solid and possesses yellow-orange color.
- The oxidation state of iron in ferrocene is ‘+2’ .
- Here the cyclopentadiene rings donates the electrons to the iron like ligands and iron forms organometallic complexes easily with the two cyclopentadiene rings.
Note: Ferrocene undergoes substitution reactions at cyclopentadiene rings and easily produces substituted products. Ferrocene has eclipsed conformation and it is stable in nature. The derivatives of ferrocene have a lot of applications in material science.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




