
Is cyclohexanol a weak acid?
Answer
510k+ views
Hint: We have to know cyclohexanol is an organic compound that has chemical formula $HOCH{\left( {C{H_2}} \right)_5}$. We have to know the common name of cyclohexanol is cyclohexyl alcohol. We have to know that an acid is a substance that donates a proton. We have to know that weak acid is a substance that does not get completely ionized when dissolved in water.
Complete answer:
We can draw the structure of cyclohexanol as,
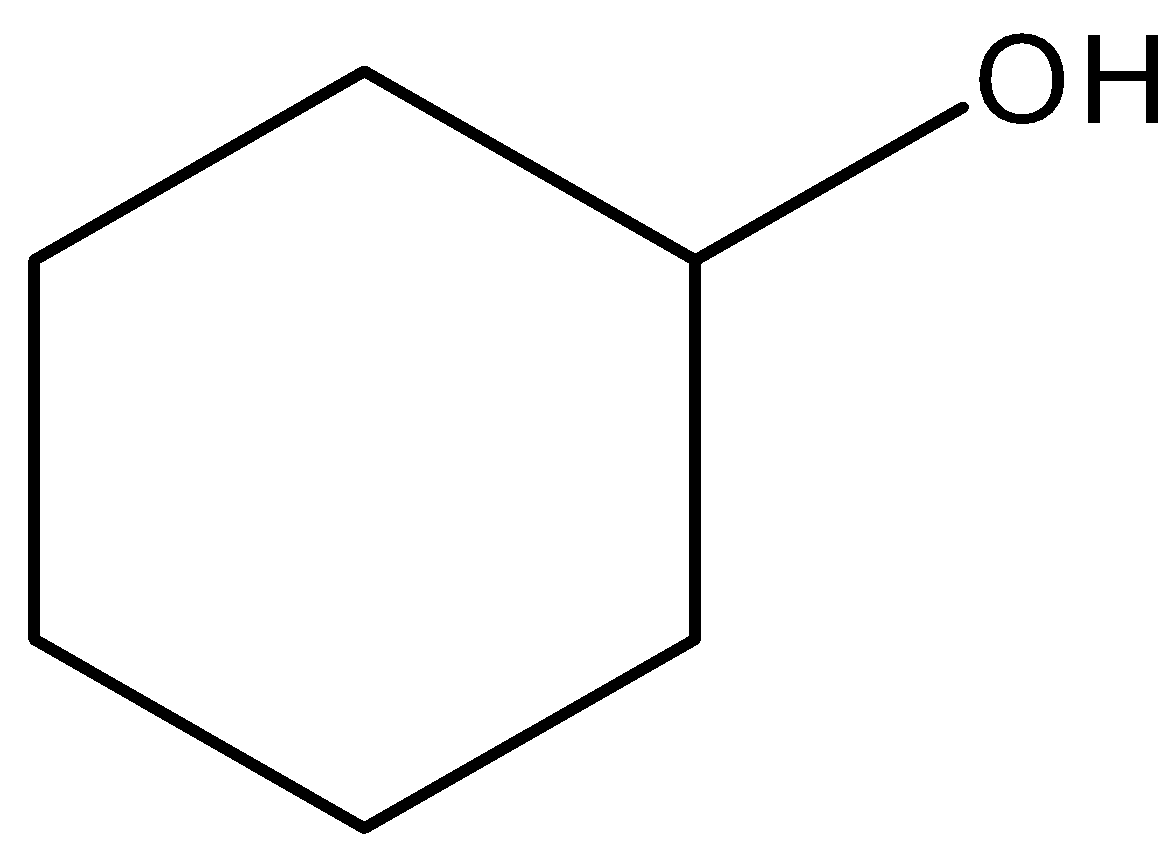
From the above structure, we can relate the molecule of cyclohexane to cyclohexanol by replacing the hydrogen atom in cyclohexane with a hydroxyl group. We have to know that cyclohexanol is a colorless solid that has a smell similar to camphor.
A weak acid is an acid that part of the way separates into its particles in an aqueous arrangement or water. Interestingly, a solid acid completely separates into its particles in water. We have to know the weak base is the conjugate base of weak acid whereas weak acid is conjugate acid of weak base. At similar concentration, the pH values of weak acids are higher when compared to stronger acids.
We can say that cyclohexanol is a weak acid because in cyclohexanol there is no delocalization of electrons. The conjugate base of cyclohexanol does not exhibit any resonance structure for the stabilization of charge and hence, its stability is less.
Cyclohexanol is a weak acid because there is no delocalization of electrons.
Note:
It is important to remember that the acidic nature of a molecule is determined if the conjugate base anion could be resonance stabilized. If the conjugate base anion is stable due to the stabilization of electrons by delocalization, the acidic nature would be more. If there is no electron delocalization, the acidic nature would be less.
Complete answer:
We can draw the structure of cyclohexanol as,

From the above structure, we can relate the molecule of cyclohexane to cyclohexanol by replacing the hydrogen atom in cyclohexane with a hydroxyl group. We have to know that cyclohexanol is a colorless solid that has a smell similar to camphor.
A weak acid is an acid that part of the way separates into its particles in an aqueous arrangement or water. Interestingly, a solid acid completely separates into its particles in water. We have to know the weak base is the conjugate base of weak acid whereas weak acid is conjugate acid of weak base. At similar concentration, the pH values of weak acids are higher when compared to stronger acids.
We can say that cyclohexanol is a weak acid because in cyclohexanol there is no delocalization of electrons. The conjugate base of cyclohexanol does not exhibit any resonance structure for the stabilization of charge and hence, its stability is less.
Cyclohexanol is a weak acid because there is no delocalization of electrons.
Note:
It is important to remember that the acidic nature of a molecule is determined if the conjugate base anion could be resonance stabilized. If the conjugate base anion is stable due to the stabilization of electrons by delocalization, the acidic nature would be more. If there is no electron delocalization, the acidic nature would be less.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




