
Is \[C{{r}_{2}}{{O}_{7}}\] a base?
Answer
552k+ views
Hint:Bases are compounds which have the tendency to liberate hydroxide ions, or donate electrons or accept protons.
These are three different definitions of base which are based on the availability of hydroxide ions, availability of electrons or unavailability or capability to accept protons.
Complete step-by-step answer:In order to answer this question at first we need to define the terms acid and bases and how can we identify both.
A substance which has the capability to donate electrons to any other species, are termed as bases. And the substances which have the tendency to accept electrons are termed as acids. Now, acid and bases can also be defined in terms of hydrogen or proton accepting and donating or liberating capabilities. If a substance liberates protons as soon as it comes in contact with an aqueous solution, then it would be termed as an acid. And if a substance can accept protons, is termed as a base.
We need to know there is something called conjugate base and conjugate acids. The conjugate base of an acid is the species which arises when an acid loses a proton and becomes negatively charged ion. So, the dichromate ion is a conjugate base of hydrogen dichromate. The structure of this species can be represented as,
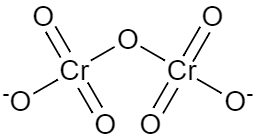
Note:So, as we can see there are seven oxygen atoms attached to two chromium atoms and two of the oxygen atoms have negative charge. Since, this ion has availability of negative charge, it can easily donate the electrons and hence it is a type of base.
These are three different definitions of base which are based on the availability of hydroxide ions, availability of electrons or unavailability or capability to accept protons.
Complete step-by-step answer:In order to answer this question at first we need to define the terms acid and bases and how can we identify both.
A substance which has the capability to donate electrons to any other species, are termed as bases. And the substances which have the tendency to accept electrons are termed as acids. Now, acid and bases can also be defined in terms of hydrogen or proton accepting and donating or liberating capabilities. If a substance liberates protons as soon as it comes in contact with an aqueous solution, then it would be termed as an acid. And if a substance can accept protons, is termed as a base.
We need to know there is something called conjugate base and conjugate acids. The conjugate base of an acid is the species which arises when an acid loses a proton and becomes negatively charged ion. So, the dichromate ion is a conjugate base of hydrogen dichromate. The structure of this species can be represented as,

Note:So, as we can see there are seven oxygen atoms attached to two chromium atoms and two of the oxygen atoms have negative charge. Since, this ion has availability of negative charge, it can easily donate the electrons and hence it is a type of base.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




