
Intramolecular hydrogen bond is present in:
(a)- Water
(b)- o-nitrophenol
(c)- p-nitrophenol
(d)- Methylamine
Answer
570k+ views
Hint: Hydrogen bonding is of two types, intermolecular hydrogen bonding, and intramolecular hydrogen bonding. Intermolecular hydrogen bonding is the bonding formed between two molecules and intramolecular hydrogen bonding is the bonding formed within the molecule.
Complete step by step answer:
There are many types of bonding involved in the formation of molecules like hydrogen bonding, van der Waal interactions, dipole-dipole interaction, etc. In the periodic table only three elements show this bonding, these are nitrogen, oxygen, and fluorine.
Hydrogen bonding is of two types, intermolecular hydrogen bonding, and intramolecular hydrogen bonding. Intermolecular hydrogen bonding is the bonding formed between two molecules and intramolecular hydrogen bonding is the bonding formed within the molecule.
So for the molecule to have intramolecular hydrogen bonding, the hydrogen atom and the electronegative atom must be near to each other in the same molecule. So from the options given, the o-nitrophenol will have intramolecular hydrogen bonding. The structure of the o-nitrophenol is given below:
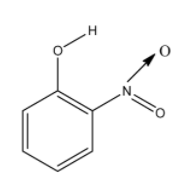
In this molecule, the nitro group and the alcohol groups are near to each other. So the hydrogen atom of the alcohol group and oxygen atom of the nitro group will form the hydrogen bonding. aThe hydrogen bonding is given below and it is denoted with dashed lines:
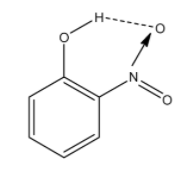
Therefore, the correct answer is an option (b)- o-nitrophenol.
Note: You may get confused that the p-nitrophenol will also have the intramolecular hydrogen bonding because it also has the nitro group and the alcohol group. But it does not show the intramolecular hydrogen bonding because the nitro group and the alcohol group are very apart for the formation of hydrogen bonding within the molecule.
Complete step by step answer:
There are many types of bonding involved in the formation of molecules like hydrogen bonding, van der Waal interactions, dipole-dipole interaction, etc. In the periodic table only three elements show this bonding, these are nitrogen, oxygen, and fluorine.
Hydrogen bonding is of two types, intermolecular hydrogen bonding, and intramolecular hydrogen bonding. Intermolecular hydrogen bonding is the bonding formed between two molecules and intramolecular hydrogen bonding is the bonding formed within the molecule.
So for the molecule to have intramolecular hydrogen bonding, the hydrogen atom and the electronegative atom must be near to each other in the same molecule. So from the options given, the o-nitrophenol will have intramolecular hydrogen bonding. The structure of the o-nitrophenol is given below:

In this molecule, the nitro group and the alcohol groups are near to each other. So the hydrogen atom of the alcohol group and oxygen atom of the nitro group will form the hydrogen bonding. aThe hydrogen bonding is given below and it is denoted with dashed lines:

Therefore, the correct answer is an option (b)- o-nitrophenol.
Note: You may get confused that the p-nitrophenol will also have the intramolecular hydrogen bonding because it also has the nitro group and the alcohol group. But it does not show the intramolecular hydrogen bonding because the nitro group and the alcohol group are very apart for the formation of hydrogen bonding within the molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




