
Inorganic benzene is:
(A) \[{B_3}{N_6}{H_3}\]
(B) \[{B_3}{N_3}{H_6}\]
(C) \[A{l_3}{N_3}{H_6}\]
(D) None of these
Answer
590.7k+ views
Hint:
The chemical name of inorganic benzene is borazine. It is a cyclic structure involving two atoms of p-block elements. It has similar chemical reactivity as benzene.
Complete step by step solution:
Here is the structure of Borazine which is known as inorganic benzene.
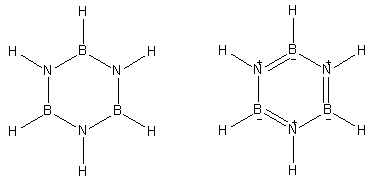
Both of the structures shown here are of borazine. It has boron, nitrogen and hydrogen atoms in its constitution.
- Borazine is known as inorganic benzene because it is made up of inorganic atoms and has similar reactivity as organic compound benzene. In its neutral structure, borazine has six hydrogen atoms directly bonded to the three nitrogen and three boron atoms.
- Borazine gives a secondary structure that involves three double bonds in the ring and positive and negative charges on nitrogen and boron atoms respectively.
- Borazine can give additional reactions as benzene can give. In fact some reactions like addition of bromine to the ring can be done without the requirement of a catalyst.
- Borazine also follows the Huckel rule, hence it is also aromatic, however it has some difference in comparison with benzene because it involves two atoms with different electronegativity.
- It does not involve Aluminium in its structure. It just has boron and nitrogen atoms arranged alternatively in the ring. So,its chemical formula is \[{{B}_{3}}{{N}_{3}}{{H}_{6}}\].
Note:
Do not consider that inorganic benzene has three hydrogen atoms as it will not fulfil the valency of the atoms. Make sure that the structure that you think of borazine should be able to give chemical reactions like benzene, the only structure possible is the answer.
The chemical name of inorganic benzene is borazine. It is a cyclic structure involving two atoms of p-block elements. It has similar chemical reactivity as benzene.
Complete step by step solution:
Here is the structure of Borazine which is known as inorganic benzene.

Both of the structures shown here are of borazine. It has boron, nitrogen and hydrogen atoms in its constitution.
- Borazine is known as inorganic benzene because it is made up of inorganic atoms and has similar reactivity as organic compound benzene. In its neutral structure, borazine has six hydrogen atoms directly bonded to the three nitrogen and three boron atoms.
- Borazine gives a secondary structure that involves three double bonds in the ring and positive and negative charges on nitrogen and boron atoms respectively.
- Borazine can give additional reactions as benzene can give. In fact some reactions like addition of bromine to the ring can be done without the requirement of a catalyst.
- Borazine also follows the Huckel rule, hence it is also aromatic, however it has some difference in comparison with benzene because it involves two atoms with different electronegativity.
- It does not involve Aluminium in its structure. It just has boron and nitrogen atoms arranged alternatively in the ring. So,its chemical formula is \[{{B}_{3}}{{N}_{3}}{{H}_{6}}\].
Note:
Do not consider that inorganic benzene has three hydrogen atoms as it will not fulfil the valency of the atoms. Make sure that the structure that you think of borazine should be able to give chemical reactions like benzene, the only structure possible is the answer.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




