
In which of the following solvent is it possible to create acetylide ions?
A. $AcOH$
B. ${H_2}O$
C. ${(i - \Pr )_2}NH$
D. Acetone
Answer
581.1k+ views
Hint: We can create an acetylide anion removing the proton from the end carbon of a terminal alkyne in presence of a strong base.
Complete step by step answer:
We have to know that the terminal alkynes are more acidic than several other hydrocarbons. Removal of the proton results in the formation of an acetylide anion $RC \equiv {C^ - }$.
The origin of the enhanced acidity could be attributed to the stability of the acetylide anion that contains the lone pair of electrons in \[sp\]hybridized orbital. The stability comes from occupying an orbital with a high degree of s-orbital character. There is a strong correlation between s-character in the orbital having the non-bonding electrons in the anion and the acidity of hydrocarbons.
Terminal alkynes are weak acids.
Acetylide anions can be formed by deprotonation using a strong base. Amide anion \[\left( {N{H_2}^ - } \right)\] in the form of $NaN{H_2}$. $NaN{H_2}$ is generally used for the formation of acetylide anions.
The chemical equation is written as,
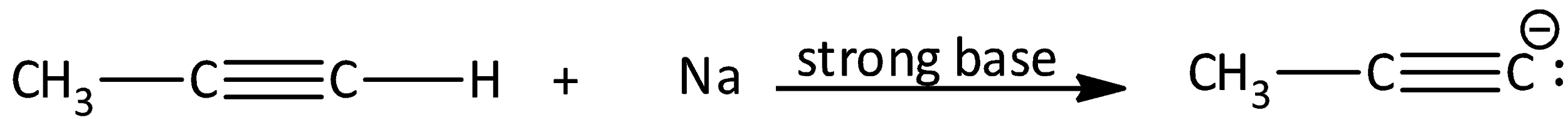
We know that acetone, water, and $AcOH$ are weaker bases than acetylide ions. Therefore, they do not react with acetylene. Therefore, Options (A), (B) and (D) are incorrect.
${(i - \Pr )_2}{N^ - }$ acts a stronger base than $RC \equiv {C^ - }$. Hence, ${(i - \Pr )_2}NH$ is used as a solvent to form acetylide ions.
So, the correct answer is Option C.
Note:
We can use acetylide as reagents in organic synthesis. We can prepare Sodium or potassium acetylides from several inorganic reagents such as sodium amide. Similarly, we can obtain silver acetylides from silver nitrate. We can Copper (I) acetylide by passing acetylene through an aqueous solution of copper (I) chloride. Acetylides act as nucleophiles that add a variety of electrophilic and unsaturated substrates. Certain acetylides are explosive.
Complete step by step answer:
We have to know that the terminal alkynes are more acidic than several other hydrocarbons. Removal of the proton results in the formation of an acetylide anion $RC \equiv {C^ - }$.
The origin of the enhanced acidity could be attributed to the stability of the acetylide anion that contains the lone pair of electrons in \[sp\]hybridized orbital. The stability comes from occupying an orbital with a high degree of s-orbital character. There is a strong correlation between s-character in the orbital having the non-bonding electrons in the anion and the acidity of hydrocarbons.
Terminal alkynes are weak acids.
Acetylide anions can be formed by deprotonation using a strong base. Amide anion \[\left( {N{H_2}^ - } \right)\] in the form of $NaN{H_2}$. $NaN{H_2}$ is generally used for the formation of acetylide anions.
The chemical equation is written as,
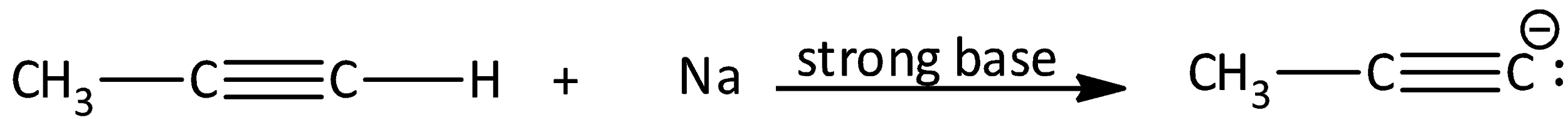
We know that acetone, water, and $AcOH$ are weaker bases than acetylide ions. Therefore, they do not react with acetylene. Therefore, Options (A), (B) and (D) are incorrect.
${(i - \Pr )_2}{N^ - }$ acts a stronger base than $RC \equiv {C^ - }$. Hence, ${(i - \Pr )_2}NH$ is used as a solvent to form acetylide ions.
So, the correct answer is Option C.
Note:
We can use acetylide as reagents in organic synthesis. We can prepare Sodium or potassium acetylides from several inorganic reagents such as sodium amide. Similarly, we can obtain silver acetylides from silver nitrate. We can Copper (I) acetylide by passing acetylene through an aqueous solution of copper (I) chloride. Acetylides act as nucleophiles that add a variety of electrophilic and unsaturated substrates. Certain acetylides are explosive.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




