
In the Rosenmund’s reaction,
$RCOCl+{{H}_{2}}\xrightarrow{Pd/BaS{{O}_{4}}}RCHO+HCl$
Here $BaS{{O}_{4}}$ :
(a)- promotes the catalytic activity of Pd
(b)- removes the HCl formed in the reaction
(c)- deactivates palladium
(d)- activates palladium
Answer
591k+ views
Hint: Acid chlorides are converted into aldehydes in the presence of palladium and barium sulfate. Palladium is a strong reducing agent. It reduces the aldehydes to further alcohol. So, it has to be stopped at the stage of the aldehydes.
Complete answer:
Acid chlorides are easily reduced to the corresponding aldehydes by passing hydrogen gas through boiling xylene solution of the acid chloride in presence of Pd catalyst supported over $BaS{{O}_{4}}$ and partially poisoned by the addition of sulfur and quinoline.
The reactions are given below:
Acid chlorides are converted into aldehydes.
$RCOCl+{{H}_{2}}\xrightarrow[Boiling\text{ }xylene]{Pd,BaS{{O}_{4}},S}RCHO+HCl$
Example: Acetyl chloride is converted into acetaldehyde.
$C{{H}_{3}}COCl+{{H}_{2}}\xrightarrow[Boiling\text{ }xylene]{Pd,BaS{{O}_{4}},S}C{{H}_{3}}CHO+HCl$
Benzoyl chloride is converted into benzaldehyde.
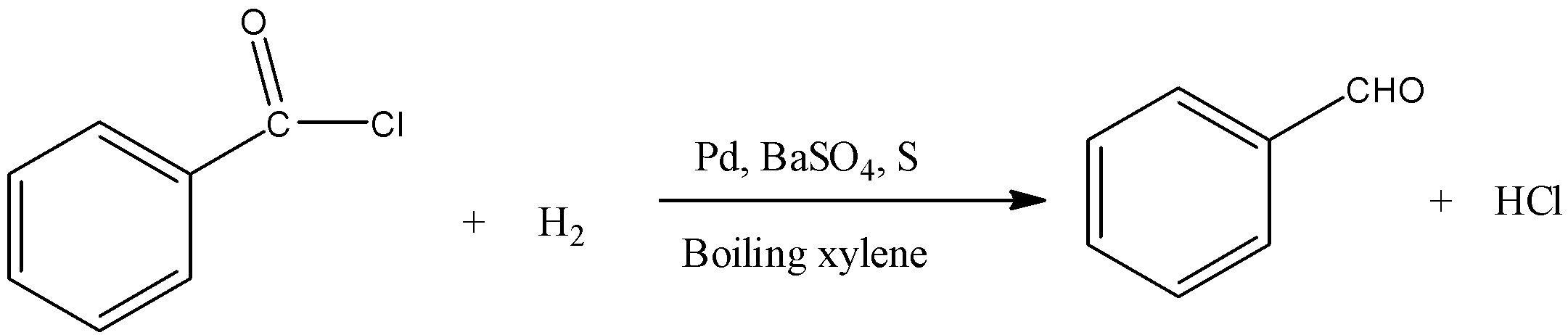
Normally, aldehydes are further reduced to primary alcohols. But the addition of $BaS{{O}_{4}}$ and sulfur (or quinoline) poisons the palladium catalyst and thus does not permit the further reduction of aldehydes to alcohols.
Hence the correct answer is an option (c)- deactivates palladium.
Additional information:
Acid chlorides can be converted into aldehydes by lithium-tert-butoxyaluminum hydride $[LiAlH{{(O-t-Bu)}_{3}}]$ at 196K.
The reaction is given below:
$RCOCl\xrightarrow[{{H}_{3}}{{O}^{+}}]{LiAlH{{(O-t-Bu)}_{3}},dry\text{ }ether,196K}RCHO$
It may be noted that $[LiAlH{{(O-t-Bu)}_{3}}]$is less reactive than $LiAl{{H}_{4}}$ because the electron-withdrawing t-butoxy group stabilizes the negatively charged aluminium ion. Therefore, it reduces the more reactive acid chlorides to aldehydes but does not reduce the less reactive aldehydes to primary alcohols.
Note: With the rosenmund reaction formaldehyde cannot be prepared since formyl chloride, $HCOCl$ is unstable to room temperature. This reaction is used only for the preparation of aldehydes but not for ketones.
Complete answer:
Acid chlorides are easily reduced to the corresponding aldehydes by passing hydrogen gas through boiling xylene solution of the acid chloride in presence of Pd catalyst supported over $BaS{{O}_{4}}$ and partially poisoned by the addition of sulfur and quinoline.
The reactions are given below:
Acid chlorides are converted into aldehydes.
$RCOCl+{{H}_{2}}\xrightarrow[Boiling\text{ }xylene]{Pd,BaS{{O}_{4}},S}RCHO+HCl$
Example: Acetyl chloride is converted into acetaldehyde.
$C{{H}_{3}}COCl+{{H}_{2}}\xrightarrow[Boiling\text{ }xylene]{Pd,BaS{{O}_{4}},S}C{{H}_{3}}CHO+HCl$
Benzoyl chloride is converted into benzaldehyde.

Normally, aldehydes are further reduced to primary alcohols. But the addition of $BaS{{O}_{4}}$ and sulfur (or quinoline) poisons the palladium catalyst and thus does not permit the further reduction of aldehydes to alcohols.
Hence the correct answer is an option (c)- deactivates palladium.
Additional information:
Acid chlorides can be converted into aldehydes by lithium-tert-butoxyaluminum hydride $[LiAlH{{(O-t-Bu)}_{3}}]$ at 196K.
The reaction is given below:
$RCOCl\xrightarrow[{{H}_{3}}{{O}^{+}}]{LiAlH{{(O-t-Bu)}_{3}},dry\text{ }ether,196K}RCHO$
It may be noted that $[LiAlH{{(O-t-Bu)}_{3}}]$is less reactive than $LiAl{{H}_{4}}$ because the electron-withdrawing t-butoxy group stabilizes the negatively charged aluminium ion. Therefore, it reduces the more reactive acid chlorides to aldehydes but does not reduce the less reactive aldehydes to primary alcohols.
Note: With the rosenmund reaction formaldehyde cannot be prepared since formyl chloride, $HCOCl$ is unstable to room temperature. This reaction is used only for the preparation of aldehydes but not for ketones.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




