
In the reaction, Y is:
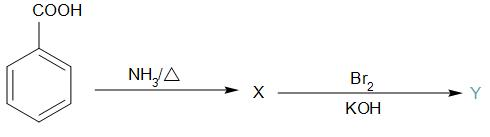
A.
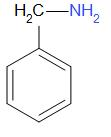
B.
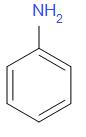
C.
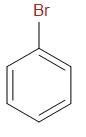
D.






Answer
569.4k+ views
Hint:To answer this question, you must recall the Hofmann bromamide degradation reaction. It is a reaction which is used to prepare primary amines from primary amides by the action of bromine in presence of an alkali like potassium hydroxide.
Complete step by step answer:
We know that Benzoic acid has a carboxyl group and is thus a weak acid. Ammonia is a weak base. An acid and a base neutralize each other and form salt and water. The first reaction involves the acid- base reaction between benzoic acid and ammonia. Benzoic acid being an acid, donates a proton to ammonia, which since is a base, accepts the proton and they form the salt ammonium benzoate.
On heating the reaction mixture, a water molecule is lost from ammonium benzoate and results in the formation of Benzamide.
When benzamide is treated with bromine in aqueous or alcoholic solution of an alkali say potassium hydroxide or sodium hydroxide, it undergoes Hofmann bromamide degradation. A primary amine is formed as a result of the reaction. In this case, aniline is the product obtained.
The product Y is
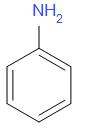
Thus, the correct answer is B.
Note:
The Hofmann bromamide reaction follows the following mechanism:
-The amide is attacked by the alkali leading to the deprotonation of the amide and forming an anion.
-Then the anion formed reacts with bromine forming N- Bromamide. This attack is said to be an alpha substitution reaction.
-Now the bromamide is deprotonated by another molecule of the base forming bromamide ion which undergoes rearrangement and an isocyanate is formed with a bromide ion leaving the molecule.
-Next the isocyanate undergoes nucleophilic addition by water forming carbamic acid. Carbamic acid being unstable loses carbon dioxide resulting into the formation of a primary amine.
Complete step by step answer:
We know that Benzoic acid has a carboxyl group and is thus a weak acid. Ammonia is a weak base. An acid and a base neutralize each other and form salt and water. The first reaction involves the acid- base reaction between benzoic acid and ammonia. Benzoic acid being an acid, donates a proton to ammonia, which since is a base, accepts the proton and they form the salt ammonium benzoate.
On heating the reaction mixture, a water molecule is lost from ammonium benzoate and results in the formation of Benzamide.
When benzamide is treated with bromine in aqueous or alcoholic solution of an alkali say potassium hydroxide or sodium hydroxide, it undergoes Hofmann bromamide degradation. A primary amine is formed as a result of the reaction. In this case, aniline is the product obtained.
The product Y is

Thus, the correct answer is B.
Note:
The Hofmann bromamide reaction follows the following mechanism:
-The amide is attacked by the alkali leading to the deprotonation of the amide and forming an anion.
-Then the anion formed reacts with bromine forming N- Bromamide. This attack is said to be an alpha substitution reaction.
-Now the bromamide is deprotonated by another molecule of the base forming bromamide ion which undergoes rearrangement and an isocyanate is formed with a bromide ion leaving the molecule.
-Next the isocyanate undergoes nucleophilic addition by water forming carbamic acid. Carbamic acid being unstable loses carbon dioxide resulting into the formation of a primary amine.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




