
In the reaction x and y are:
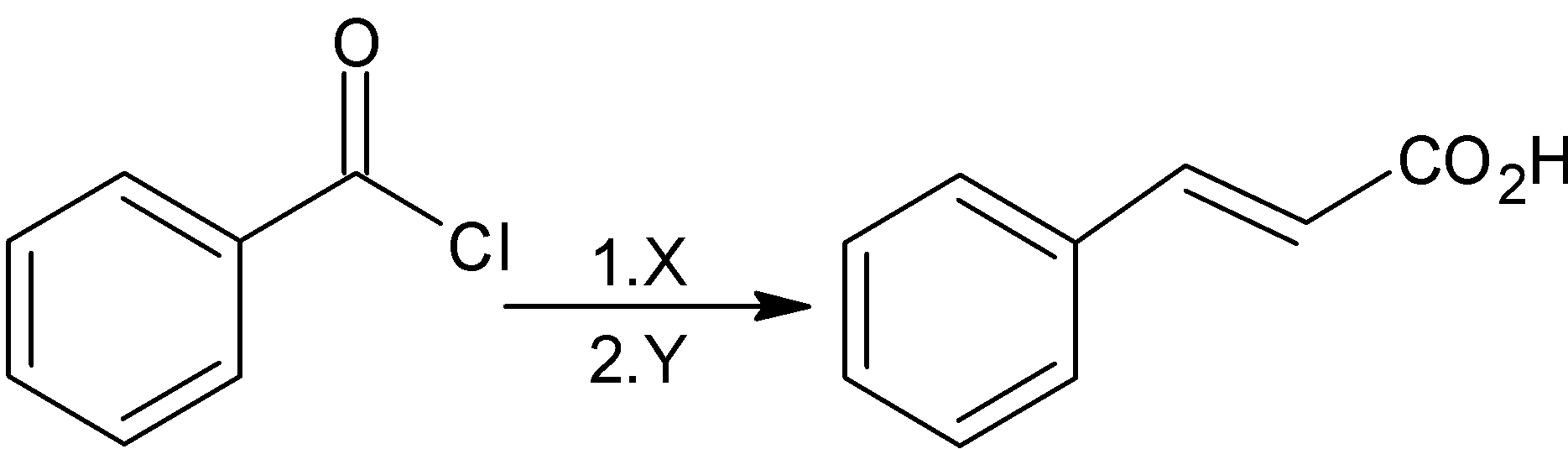
A)\[\text{x = }{{\text{H}}_{\text{2}}}\text{,Pd/BaS}{{\text{O}}_{\text{4}}}\text{ ; y = NaOAc, A}{{\text{c}}_{\text{2}}}\text{O}\]
B)\[\text{x = LiAl}{{\text{H}}_{\text{4}}}\text{ ; y = NaOAc, A}{{\text{c}}_{\text{2}}}\text{O}\]
C)\[\text{X = }{{\text{H}}_{\text{2}}}\text{, Pd/C ; y = NaOH, A}{{\text{c}}_{\text{2}}}\text{O}\]
D)\[\text{x = LiAl}{{\text{H}}_{\text{4}}}\text{ ; y = NaOH, }\!\!~\!\!\text{ A}{{\text{c}}_{\text{2}}}\text{O}\]

Answer
592.2k+ views
Hint: An acyl chloride is converted into the aldehydes through the Rosenmunds reduction reaction. These reactions are followed by the Perkin reaction. In the Perkin reaction, aldehydes are converted into the $\text{ }\!\!\alpha\!\!\text{ , }\!\!\beta\!\!\text{ -}$unsaturated carboxylic acid.
Complete step by step answer:
Rosenmund reduction is the reduction of acyl chlorides \[\text{ROCl}\]into the aldehyde$\text{RCHO}$. The acyl chloride undergoes the hydrogenation (addition of ${{\text{H}}_{\text{2}}}$) in presence of palladium $\text{Pd}$catalysts on the surface of barium sulfate $\text{(BaS}{{\text{O}}_{\text{4}}}\text{)}$ to get the corresponding aldehydes. This is Rosenmund's reaction. The barium sulfate prevents the further reduction of aldehydes into the alcohols.
The general reaction is as follows:
\[\begin{align}
& \text{R-COCl}\xrightarrow[\text{Pd/BaS}{{\text{O}}_{\text{4}}}]{{{\text{H}}_{\text{2}}}}\text{R-CHO} \\
& \text{Acid Aldehyde} \\
& \text{Chloride} \\
\end{align}\]
Where R can be alkyl or the aryl group.
Thus, when the benzoyl chloride $\text{(}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{COCl)}$ undergoes the Rosenmund’s reduction reaction with the hydrogen and palladium on the surface of barium sulfate We get the benzaldehyde $\text{(}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CHO)}$ as the product.

The perkin reaction is a reaction used for the preparation of cinnamic acid from the benzaldehyde. The reaction involves the conversion of aromatic aldehydes $\text{(Ar-CHO)}$ to the $\text{ }\!\!\alpha\!\!\text{ , }\!\!\beta\!\!\text{ -}$unsaturated carboxylic acid by the treatment of acetic anhydride\[\text{(A}{{\text{c}}_{\text{2}}}\text{O)}\] in presence of sodium acetate as a base. The unsaturated carboxylic acid form is either E or Z. The general reaction is as follows:
$\text{Ar-CHO}\xrightarrow[\text{NaoAc}]{\text{A}{{\text{c}}_{\text{2}}}\text{O}}\text{ArCH=CHCOOH}$
Under the presence of a base, the anhydride loses the proton and form carbanion. This carbanion attacks on the carbonyl carbon of the aldehyde. The product is further hydrolysed to give the unsaturated acid.
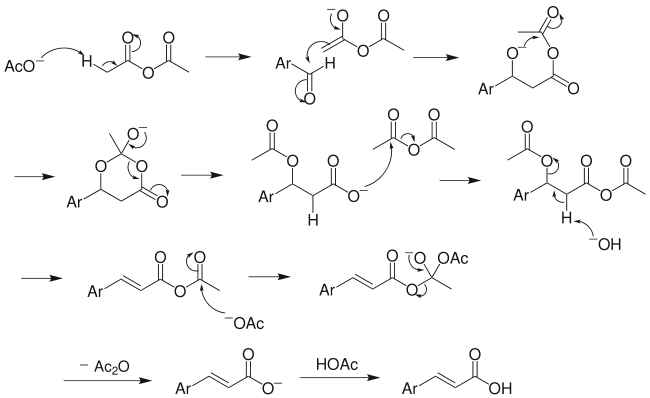
Here the benzaldehyde obtained through the Rosenmund reduction reaction undergoes the reaction with acetic anhydride in presence of sodium acetate to give cinnamic acid as follows:
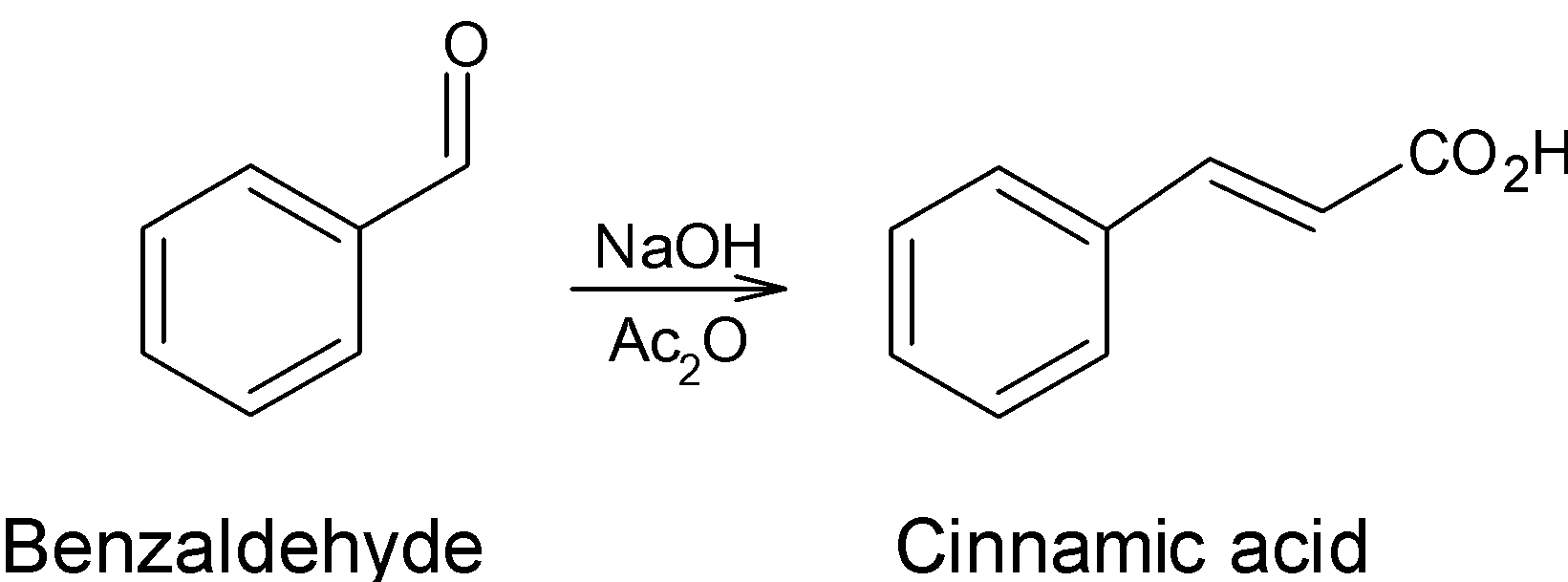
Thus, we were able to convert the benzoyl into the $\text{ }\!\!\alpha\!\!\text{ , }\!\!\beta\!\!\text{ -}$ unsaturated carboxylic acid i.e. cinnamic acid by the reduction of a benzoyl chloride by the palladium on barium sulfate which is Rosenmund reduction followed by the treatment of acetic anhydride which is Perkin reaction. Therefore, in the conversion
\[\begin{align}
& \text{X=}{{\text{H}}_{\text{2}}}\text{,Pd/BaS}{{\text{O}}_{\text{4}}} \\
& \text{Y=NaOAc, A}{{\text{c}}_{\text{2}}}\text{O} \\
\end{align}\]
So, the correct answer is “Option A”.
Note: Lithium aluminium hydride $\text{LiAl}{{\text{H}}_{\text{4}}}$ converts the acyl chloride into the primary alcohol. Therefore it is not advisable to use. Barium sulfate prevents the reaction from undergoing further reduction for alcohol. It is a poison for the palladium catalyst.
Complete step by step answer:
Rosenmund reduction is the reduction of acyl chlorides \[\text{ROCl}\]into the aldehyde$\text{RCHO}$. The acyl chloride undergoes the hydrogenation (addition of ${{\text{H}}_{\text{2}}}$) in presence of palladium $\text{Pd}$catalysts on the surface of barium sulfate $\text{(BaS}{{\text{O}}_{\text{4}}}\text{)}$ to get the corresponding aldehydes. This is Rosenmund's reaction. The barium sulfate prevents the further reduction of aldehydes into the alcohols.
The general reaction is as follows:
\[\begin{align}
& \text{R-COCl}\xrightarrow[\text{Pd/BaS}{{\text{O}}_{\text{4}}}]{{{\text{H}}_{\text{2}}}}\text{R-CHO} \\
& \text{Acid Aldehyde} \\
& \text{Chloride} \\
\end{align}\]
Where R can be alkyl or the aryl group.
Thus, when the benzoyl chloride $\text{(}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{COCl)}$ undergoes the Rosenmund’s reduction reaction with the hydrogen and palladium on the surface of barium sulfate We get the benzaldehyde $\text{(}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CHO)}$ as the product.

The perkin reaction is a reaction used for the preparation of cinnamic acid from the benzaldehyde. The reaction involves the conversion of aromatic aldehydes $\text{(Ar-CHO)}$ to the $\text{ }\!\!\alpha\!\!\text{ , }\!\!\beta\!\!\text{ -}$unsaturated carboxylic acid by the treatment of acetic anhydride\[\text{(A}{{\text{c}}_{\text{2}}}\text{O)}\] in presence of sodium acetate as a base. The unsaturated carboxylic acid form is either E or Z. The general reaction is as follows:
$\text{Ar-CHO}\xrightarrow[\text{NaoAc}]{\text{A}{{\text{c}}_{\text{2}}}\text{O}}\text{ArCH=CHCOOH}$
Under the presence of a base, the anhydride loses the proton and form carbanion. This carbanion attacks on the carbonyl carbon of the aldehyde. The product is further hydrolysed to give the unsaturated acid.

Here the benzaldehyde obtained through the Rosenmund reduction reaction undergoes the reaction with acetic anhydride in presence of sodium acetate to give cinnamic acid as follows:

Thus, we were able to convert the benzoyl into the $\text{ }\!\!\alpha\!\!\text{ , }\!\!\beta\!\!\text{ -}$ unsaturated carboxylic acid i.e. cinnamic acid by the reduction of a benzoyl chloride by the palladium on barium sulfate which is Rosenmund reduction followed by the treatment of acetic anhydride which is Perkin reaction. Therefore, in the conversion
\[\begin{align}
& \text{X=}{{\text{H}}_{\text{2}}}\text{,Pd/BaS}{{\text{O}}_{\text{4}}} \\
& \text{Y=NaOAc, A}{{\text{c}}_{\text{2}}}\text{O} \\
\end{align}\]
So, the correct answer is “Option A”.
Note: Lithium aluminium hydride $\text{LiAl}{{\text{H}}_{\text{4}}}$ converts the acyl chloride into the primary alcohol. Therefore it is not advisable to use. Barium sulfate prevents the reaction from undergoing further reduction for alcohol. It is a poison for the palladium catalyst.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




