
In the Lewis structure of $ICl_2^ -$, how many lone pairs of electrons are around the iodine atom?
Answer
558.6k+ views
Hint: In $ICl_2^ -$, the central atom is iodine which is surrounded by two chlorine atoms. The electronic configuration of iodine is $[Kr]4{d^{10}}5{s^2}5{p^5}$and the electronic configuration of chlorine is $[Ne]3{s^2}3{p^5}$.
Complete step by step answer:
Lewis structure is defined as the representation for the arrangement of valence electrons surrounding each atom present in the molecule.
The compound $ICl_2^ -$ is an anion, where the central iodine atom is attached with two chlorine atoms by a single bond and a negative charge is present on the overall molecule. The atomic number of iodine is 53 and the electronic configuration of iodine is $[Kr]4{d^{10}}5{s^2}5{p^5}$. The valence electrons in iodine is 7 where two electrons take part in bonding and six electrons are present as three lone pairs. The atomic number of chlorine is 17 and the electronic configuration of chlorine is $[Ne]3{s^2}3{p^5}$. The valence electrons in chlorine is 7. As two chlorine atoms are present one electron from each chlorine takes part in bonding and each chlorine atom contains 6 electrons as three lone pairs. The negative charge shows that one electron is gained by iodine atom.
The Lewis structure of $ICl_2^ -$ is shown below.
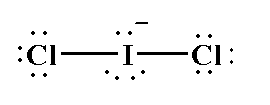
Therefore, In the Lewis structure of $ICl_2^ -$, 3 lone pairs of electrons are around the iodine atom.
Note:
The relative atomic mass has no units. The calculation of relative formula mass is done the same as the calculation of molecular weight where atomic mass of the atoms are added but the unit of molecular weight is g/mol.
Complete step by step answer:
Lewis structure is defined as the representation for the arrangement of valence electrons surrounding each atom present in the molecule.
The compound $ICl_2^ -$ is an anion, where the central iodine atom is attached with two chlorine atoms by a single bond and a negative charge is present on the overall molecule. The atomic number of iodine is 53 and the electronic configuration of iodine is $[Kr]4{d^{10}}5{s^2}5{p^5}$. The valence electrons in iodine is 7 where two electrons take part in bonding and six electrons are present as three lone pairs. The atomic number of chlorine is 17 and the electronic configuration of chlorine is $[Ne]3{s^2}3{p^5}$. The valence electrons in chlorine is 7. As two chlorine atoms are present one electron from each chlorine takes part in bonding and each chlorine atom contains 6 electrons as three lone pairs. The negative charge shows that one electron is gained by iodine atom.
The Lewis structure of $ICl_2^ -$ is shown below.

Therefore, In the Lewis structure of $ICl_2^ -$, 3 lone pairs of electrons are around the iodine atom.
Note:
The relative atomic mass has no units. The calculation of relative formula mass is done the same as the calculation of molecular weight where atomic mass of the atoms are added but the unit of molecular weight is g/mol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




