
In the given transformation, which of the following is the most appropriate reagent?
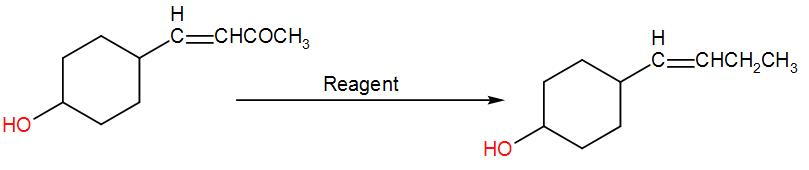
[A] $N{{H}_{2}}N{{H}_{2}}\text{, O}{{\text{H}}^{-}}$
[B] $Zn-Hg/HCl$
[C] Na, liq.$N{{H}_{3}}$
[D] $NaB{{H}_{4}}$

Answer
566.7k+ views
Hint:To solve this firstly identify the functional group that is changing here. Here –CO is reduced to an alkane. The reaction will take place in basic condition and a hydrazone intermediate will be formed. You can use this to answer this question.
Complete step by step solution:
In the given question, firstly we have to understand that here –CO is reduced to $C{{H}_{2}}$ and the rest of the structure is kept intact. In the options four different reagents are given to us that will affect the reactant in different ways. SO, we will go through each option and try to find out the appropriate reagent.
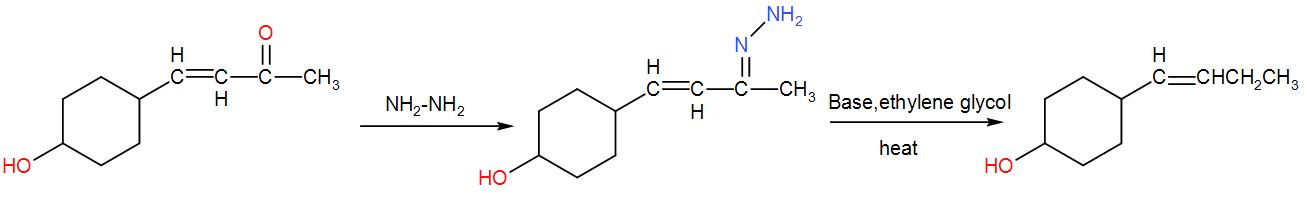
Firstly, we have $N{{H}_{2}}N{{H}_{2}}\text{, O}{{\text{H}}^{-}}$. This reagent is used in Wolff-Kishner reduction where the carbonyl functional group is converted to methylene group. Upon addition of $N{{H}_{2}}-N{{H}_{2}}$ we get hydrazine by the attack of the carbonyl group. It is further reduced to an alkane by addition of ethylene glycol and heating. We can write the reaction as-
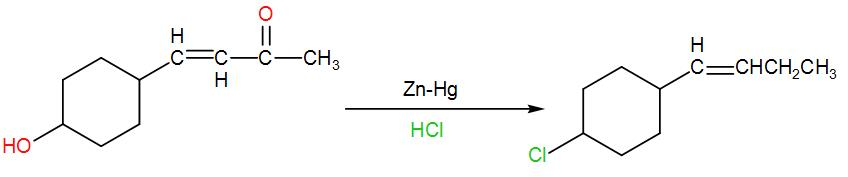
In the next option we have $Zn-Hg/HCl$. We use this reagent for reduction of carbonyl groups. Here also the carbonyl group will be reduced and will give us an alkane but here the solution is acidic. This acidic condition will cause protonation of the –OH group and then finally it will be substituted by chlorine. We can write the reaction as-
Then we have Na i.e. sodium in liquid ammonia. This reagent is used for conversion of alkyne to alkenes but alkenes are not converted to alkanes by this reagent. Therefore, this can’t be the appropriate reagent.
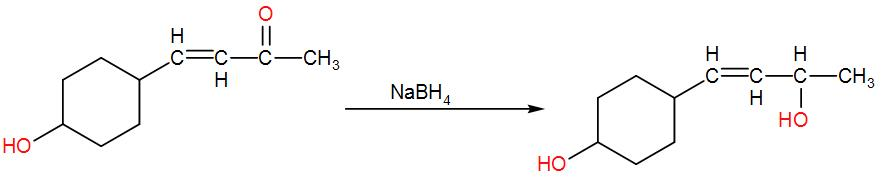
And lastly we have $NaB{{H}_{4}}$. We use sodium borohydride for reduction of –CO to –COH. Here, it will reduce the carbonyl group –CO to –CHOH. We can write the reaction as-
We can understand from the above discussion that the appropriate reagent is $N{{H}_{2}}N{{H}_{2}}\text{, O}{{\text{H}}^{-}}$.
Therefore, the correct answer is option [A] $N{{H}_{2}}N{{H}_{2}}\text{, O}{{\text{H}}^{-}}$.
Note: Here, if we had a nitro group instead of the hydroxyl group, the Clemmensen reagent i.e. zinc amalgam, would reduce the nitro group to an amine. However, in Wolff – Kishner reduction, there would be no additional reduction step after hydrolysis. However, if we did not have any acid sensitive group present then we could’ve used either of the two for the reduction.
Complete step by step solution:
In the given question, firstly we have to understand that here –CO is reduced to $C{{H}_{2}}$ and the rest of the structure is kept intact. In the options four different reagents are given to us that will affect the reactant in different ways. SO, we will go through each option and try to find out the appropriate reagent.
Firstly, we have $N{{H}_{2}}N{{H}_{2}}\text{, O}{{\text{H}}^{-}}$. This reagent is used in Wolff-Kishner reduction where the carbonyl functional group is converted to methylene group. Upon addition of $N{{H}_{2}}-N{{H}_{2}}$ we get hydrazine by the attack of the carbonyl group. It is further reduced to an alkane by addition of ethylene glycol and heating. We can write the reaction as-

In the next option we have $Zn-Hg/HCl$. We use this reagent for reduction of carbonyl groups. Here also the carbonyl group will be reduced and will give us an alkane but here the solution is acidic. This acidic condition will cause protonation of the –OH group and then finally it will be substituted by chlorine. We can write the reaction as-

Then we have Na i.e. sodium in liquid ammonia. This reagent is used for conversion of alkyne to alkenes but alkenes are not converted to alkanes by this reagent. Therefore, this can’t be the appropriate reagent.
And lastly we have $NaB{{H}_{4}}$. We use sodium borohydride for reduction of –CO to –COH. Here, it will reduce the carbonyl group –CO to –CHOH. We can write the reaction as-

We can understand from the above discussion that the appropriate reagent is $N{{H}_{2}}N{{H}_{2}}\text{, O}{{\text{H}}^{-}}$.
Therefore, the correct answer is option [A] $N{{H}_{2}}N{{H}_{2}}\text{, O}{{\text{H}}^{-}}$.
Note: Here, if we had a nitro group instead of the hydroxyl group, the Clemmensen reagent i.e. zinc amalgam, would reduce the nitro group to an amine. However, in Wolff – Kishner reduction, there would be no additional reduction step after hydrolysis. However, if we did not have any acid sensitive group present then we could’ve used either of the two for the reduction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




