
In the following reaction, compound Z Is:
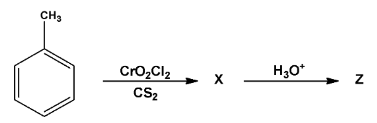
\[A.\;\,\,Benzoic{\text{ }}acid\]
\[B.\;\,\,Benzaldehyde\]
\[C.\,\,Acetophenone\]
\[D.\,\,benzene\]

Answer
546k+ views
Hint:It is a type of an Etard’s reaction which involves the conversion of alkyl benzene or compounds having alkyl group attached to a heterocyclic ring into aromatic aldehydes in presence of chromyl chloride. In this case, we have toluene which reacts with chromyl chloride in the presence of carbon disulfide as a medium forms Etard complex then acidic hydrolysis takes place to form benzaldehyde. This reaction is an Etard reaction.
Complete step-by-step answer:The Etard reaction is one type of reaction in which the direct oxidation of an aromatic or heterocyclic bound alkyl group takes place. In this reaction mechanism, via ene-reaction, the further reaction takes place with the chromyl chloride that forms a precipitate i.e., an Etard complex. The Etard complex which was formed will further decompose by sigma-tropic rearrangement under reducing conditions which prevent the further oxidation to a carboxylic acid. The main reducing condition is provided by a saturated solution of aqueous sodium sulfite \[\left( {N{a_2}S{O_3}} \right)\] which prevents further decomposition.
In the aforesaid reaction, the toluene \[({C_6}{H_5}C{H_3})\] reacts with chromyl chloride in the presence of carbon disulfide as a medium form the Etard complex. This complex gets reduced to in the presence of acidic medium (where acidic hydrolysis takes place) to form benzaldehyde.
In this reaction,to chromyl chloride \[\;\left( {Cr{O_2}C{l_2}} \right)\] attacks on the methyl group connected to the benzene ring to form an intermediate which further undergoes \[2-3\,sigmatropic\] rearrangement reaction to form a chromium complex, and this complex undergoes hydrolyses so easily to benzaldehyde as a product.
The reaction goes like;
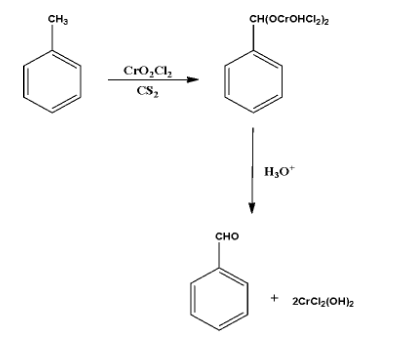
The toluene \[({C_6}{H_5}C{H_3})\] reacts with chromyl chloride \[\;\left( {Cr{O_2}C{l_2}} \right)\] in the presence of carbon disulfide \[\;\left( {C{S_2}} \right)\] as a medium forms Etard complex which is further reacted with the hydronium ion \[\;\left( {{H_3}{O^ + }} \right)\] and form benzaldehyde \[({C_6}{H_5}CHO)\]. This reaction is an Etard reaction.
Toluene \[({C_6}{H_5}C{H_3})\] is oxidized with alkaline potassium permanganate $(KMn{O_4})$ followed by acidification to form benzoic acid which on further heating with soda-lime that results in the formation of benzene. The oxidation of toluene to benzoic acid \[({C_6}{H_5}COOH)\] and then converted to benzoyl chloride \[({C_6}{H_5}COCl)\] by treating with thionyl chloride \[\left( {SOC{l_2}} \right)\] and then treated with methyl lithium \[\left( {C{H_3}Li} \right)\] to form Acetophenone \[({C_6}{H_5}COC{H_3})\] .
So, the correct answer for this question is benzaldehyde formation is taking place.
Therefore, the option \[B.\;\,\,Benzaldehyde\]\[({C_6}{H_5}CHO)\] is the correct answer.
Note:The chromyl chloride \[\;\left( {Cr{O_2}C{l_2}} \right)\] is used to form an organic complex Etard Complex which can undergo hydrolysis to form the desired compound. Benzaldehyde \[({C_6}{H_5}CHO)\] can be reduced to toluene \[({C_6}{H_5}C{H_3})\] by Clemmenson reduction reaction.
Complete step-by-step answer:The Etard reaction is one type of reaction in which the direct oxidation of an aromatic or heterocyclic bound alkyl group takes place. In this reaction mechanism, via ene-reaction, the further reaction takes place with the chromyl chloride that forms a precipitate i.e., an Etard complex. The Etard complex which was formed will further decompose by sigma-tropic rearrangement under reducing conditions which prevent the further oxidation to a carboxylic acid. The main reducing condition is provided by a saturated solution of aqueous sodium sulfite \[\left( {N{a_2}S{O_3}} \right)\] which prevents further decomposition.
In the aforesaid reaction, the toluene \[({C_6}{H_5}C{H_3})\] reacts with chromyl chloride in the presence of carbon disulfide as a medium form the Etard complex. This complex gets reduced to in the presence of acidic medium (where acidic hydrolysis takes place) to form benzaldehyde.
In this reaction,to chromyl chloride \[\;\left( {Cr{O_2}C{l_2}} \right)\] attacks on the methyl group connected to the benzene ring to form an intermediate which further undergoes \[2-3\,sigmatropic\] rearrangement reaction to form a chromium complex, and this complex undergoes hydrolyses so easily to benzaldehyde as a product.
The reaction goes like;

The toluene \[({C_6}{H_5}C{H_3})\] reacts with chromyl chloride \[\;\left( {Cr{O_2}C{l_2}} \right)\] in the presence of carbon disulfide \[\;\left( {C{S_2}} \right)\] as a medium forms Etard complex which is further reacted with the hydronium ion \[\;\left( {{H_3}{O^ + }} \right)\] and form benzaldehyde \[({C_6}{H_5}CHO)\]. This reaction is an Etard reaction.
Toluene \[({C_6}{H_5}C{H_3})\] is oxidized with alkaline potassium permanganate $(KMn{O_4})$ followed by acidification to form benzoic acid which on further heating with soda-lime that results in the formation of benzene. The oxidation of toluene to benzoic acid \[({C_6}{H_5}COOH)\] and then converted to benzoyl chloride \[({C_6}{H_5}COCl)\] by treating with thionyl chloride \[\left( {SOC{l_2}} \right)\] and then treated with methyl lithium \[\left( {C{H_3}Li} \right)\] to form Acetophenone \[({C_6}{H_5}COC{H_3})\] .
So, the correct answer for this question is benzaldehyde formation is taking place.
Therefore, the option \[B.\;\,\,Benzaldehyde\]\[({C_6}{H_5}CHO)\] is the correct answer.
Note:The chromyl chloride \[\;\left( {Cr{O_2}C{l_2}} \right)\] is used to form an organic complex Etard Complex which can undergo hydrolysis to form the desired compound. Benzaldehyde \[({C_6}{H_5}CHO)\] can be reduced to toluene \[({C_6}{H_5}C{H_3})\] by Clemmenson reduction reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




