
In the cyclo-${{S}_{8}}$ molecule of rhombic sulphur, all the S-S bond lengths and all the S-S-S bond angles are respectively (give approximate values)
(A) 204 pm and ${{105}^{{}^\circ }}$
(B) 102 pm ${{120}^{{}^\circ }}$
(C) 204 pm ${{180}^{{}^\circ }}$
(D) 102 pm ${{60}^{{}^\circ }}$
Answer
582.6k+ views
Hint: Sulphur forms numerous allotropes, but let us study the two most important allotropes of sulphur-yellow rhombic sulphur ($\alpha $-sulphur) and the monoclinic ($\beta $-sulphur). The allotropes of sulphur are inter-convertible i.e. rhombic sulphur when heated above 369K gives monoclinic sulphur.
Complete step by step answer:
Octasulfur is an inorganic chemical with the chemical formula ${{S}_{8}}$. It is a yellow solid, and is odourless and tasteless. It is the most common allotrope of sulfur. It is a major industrial chemical that occurs widely in nature.
${{S}_{8}}$ has a puckered ring structure where each sulphur atom is bonded with two other atoms forming a ring. In ${{S}_{8}}$the bond angle between two bonds of one sulphur atom is relatively small and they can withstand because of the larger size of sulphur atom which makes repulsion between them weaker.
${{S}_{8}}$ molecule has eight single covalent bonds among eight S atoms and form a closed crown shaped structure.The structure of ${{S}_{8}}$ molecule is as follows:
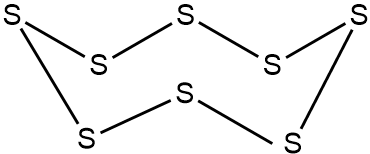
In all of the ring forms of S, the S-S distance is 204 pm and the bond angle of S-S-S is ${{105}^{{}^\circ }}$.
So, the correct answer is “Option A”.
Note: They are all soluble in $C{{S}_{2}}$. The Orthorhombic form of sulphur is thermodynamically and most stable. Rhombic sulphur is prepared by dissolving powdered sulphur in carbon disulphide at room temperature. The mixture is then filtered. The filtrate is then kept in a small beaker covered with a filter paper.
Complete step by step answer:
Octasulfur is an inorganic chemical with the chemical formula ${{S}_{8}}$. It is a yellow solid, and is odourless and tasteless. It is the most common allotrope of sulfur. It is a major industrial chemical that occurs widely in nature.
${{S}_{8}}$ has a puckered ring structure where each sulphur atom is bonded with two other atoms forming a ring. In ${{S}_{8}}$the bond angle between two bonds of one sulphur atom is relatively small and they can withstand because of the larger size of sulphur atom which makes repulsion between them weaker.
${{S}_{8}}$ molecule has eight single covalent bonds among eight S atoms and form a closed crown shaped structure.The structure of ${{S}_{8}}$ molecule is as follows:

In all of the ring forms of S, the S-S distance is 204 pm and the bond angle of S-S-S is ${{105}^{{}^\circ }}$.
So, the correct answer is “Option A”.
Note: They are all soluble in $C{{S}_{2}}$. The Orthorhombic form of sulphur is thermodynamically and most stable. Rhombic sulphur is prepared by dissolving powdered sulphur in carbon disulphide at room temperature. The mixture is then filtered. The filtrate is then kept in a small beaker covered with a filter paper.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




