
In given reaction, product B is:
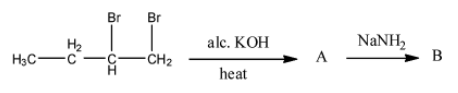
A. But-2-ene
B. Butyne
C. But-2-yne
D. Butene

Answer
570k+ views
Hint: Generally alcoholic KOH acts as a strong base and removes the acidic hydrogen from the organic compounds easily. Alkyl halides undergo reaction with alcoholic KOH and form a dehydro halogenated compound as the product.
Complete step by step answer:
- In the question it is given what is the product B in the given reaction.
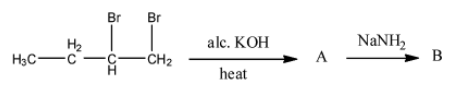
- In the given reaction there are two steps.
Step-1:
- We know that alc. KOH acts as base and removes the acidic hydrogens present in the given compounds.
- The chemical reaction of 1,2-dibromo butane with alc. KOH is as follows.
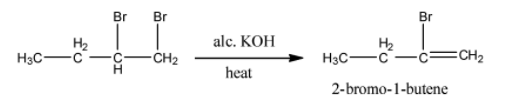
- The product formed in the above chemical reaction contains a double bond in between carbon-1 and carbon-2.
- The name of the compound formed in step-1 is 2-bromo-1-butene.
Step-2:
- The product formed in the step-1 reacts with sodamide and the chemical reaction is as follows.
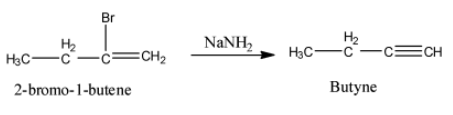
- In the above chemical reaction 2-bromo-1-butene reacts with sodamide and forms a product called butyne.
- The total given chemical reaction is as follows.
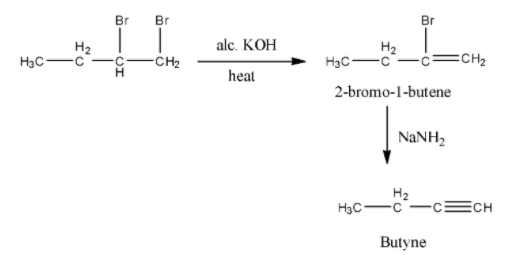
- There the product B formed in the given reaction is Butyne.
So, the correct option is B.
Note: Alcoholic $KOH$ selectively removes the acidic hydrogens present in the given compound. Alc. $KOH$ is a strong base when compared to normal potassium hydroxide ($KOH$). If the organic compound does not contain acidic hydrogens then there is no action of alc. $KOH$ on it. In organic chemistry alc. $KOH$ has a good role in synthesis.
Complete step by step answer:
- In the question it is given what is the product B in the given reaction.

- In the given reaction there are two steps.
Step-1:
- We know that alc. KOH acts as base and removes the acidic hydrogens present in the given compounds.
- The chemical reaction of 1,2-dibromo butane with alc. KOH is as follows.

- The product formed in the above chemical reaction contains a double bond in between carbon-1 and carbon-2.
- The name of the compound formed in step-1 is 2-bromo-1-butene.
Step-2:
- The product formed in the step-1 reacts with sodamide and the chemical reaction is as follows.

- In the above chemical reaction 2-bromo-1-butene reacts with sodamide and forms a product called butyne.
- The total given chemical reaction is as follows.

- There the product B formed in the given reaction is Butyne.
So, the correct option is B.
Note: Alcoholic $KOH$ selectively removes the acidic hydrogens present in the given compound. Alc. $KOH$ is a strong base when compared to normal potassium hydroxide ($KOH$). If the organic compound does not contain acidic hydrogens then there is no action of alc. $KOH$ on it. In organic chemistry alc. $KOH$ has a good role in synthesis.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




