
In ${\text{CsCl}}$ type structure, the coordination number of ${\text{C}}{{\text{s}}^ + }$ and ${\text{C}}{{\text{l}}^ - }$ respectively are:
A. $6,6$
B. $6,8$
C. $8,8$
D. $8,6$
Answer
584.4k+ views
Hint:
Solids are of two types: crystalline and amorphous. ${\text{CsCl}}$ is an example of ionic crystals which are the crystals containing ions in the lattice point. Coordination number is the number of its neighboring particles in a crystal. ${\text{CsCl}}$ is involved in the body centered cube type of cubic lattice.
Complete step by step answer:
Depending upon their structure, solids are classified into crystalline and amorphous. Crystalline solids are generally anisotropic. They are considered as true solids. Crystal lattice is a highly ordered three-dimensional structure, formed by its constituent atom or molecule or ions. Lattice is an infinite set of points repeated regularly throughout space. Unit cell is the smallest building unit in the crystal. The unit cell is characterized by length and angle. They are known as unit cell parameters. Depending upon the variation in these cell parameters, crystal lattice are of seven types.
In a cubic crystal, the intercept on three axes is equal and the angle is ${90^ \circ }$. Cubic crystals are of three types-simple, body-centered and face-centered.
${\text{CsCl}}$ is an example of body-centered crystal lattice. In this lattice, an atom in the center of the cell has all the eight corner atoms as its close neighbors. Hence the coordination number is eight.
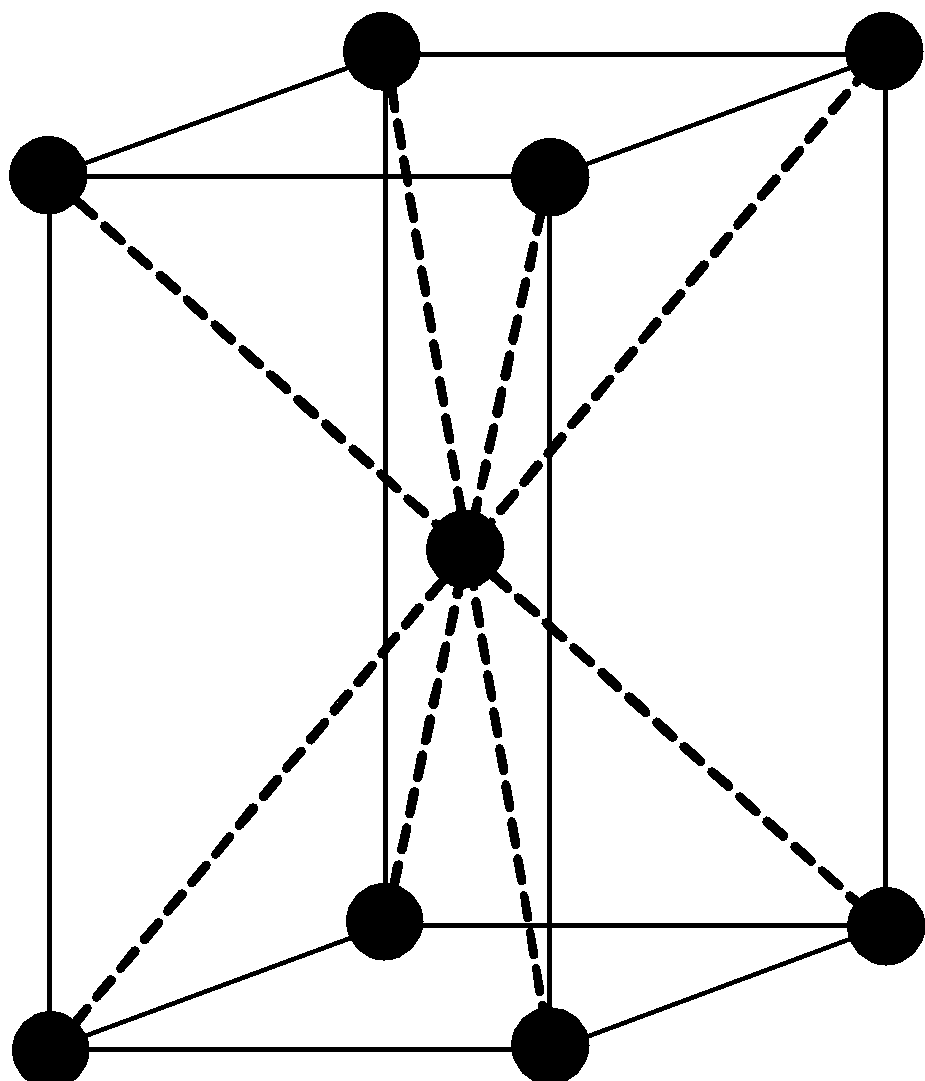
Given below is the unit cell structure of ${\text{CsCl}}$. The ion in the center is the ${\text{C}}{{\text{s}}^ + }$ ion and it is surrounded by eight ${\text{C}}{{\text{l}}^ - }$ ions at the corners. Each ${\text{C}}{{\text{l}}^ - }$ ion is also surrounded by eight ${\text{C}}{{\text{s}}^ + }$ ions. Thus both have coordination numbers $8$.
Hence the correct option is C.
Note:
In bcc lattice, there are lattice points at eight corners and at the center of the unit cell. The atom at the center is independent of other cells, while each of eight atoms situated at the corners is shared by a total of eight unit cells. Thus the total number of atoms per unit cell$ = 1 + 8 \times \dfrac{1}{8} = 2$. So there are two atoms per unit cell of bcc.
Solids are of two types: crystalline and amorphous. ${\text{CsCl}}$ is an example of ionic crystals which are the crystals containing ions in the lattice point. Coordination number is the number of its neighboring particles in a crystal. ${\text{CsCl}}$ is involved in the body centered cube type of cubic lattice.
Complete step by step answer:
Depending upon their structure, solids are classified into crystalline and amorphous. Crystalline solids are generally anisotropic. They are considered as true solids. Crystal lattice is a highly ordered three-dimensional structure, formed by its constituent atom or molecule or ions. Lattice is an infinite set of points repeated regularly throughout space. Unit cell is the smallest building unit in the crystal. The unit cell is characterized by length and angle. They are known as unit cell parameters. Depending upon the variation in these cell parameters, crystal lattice are of seven types.
In a cubic crystal, the intercept on three axes is equal and the angle is ${90^ \circ }$. Cubic crystals are of three types-simple, body-centered and face-centered.
${\text{CsCl}}$ is an example of body-centered crystal lattice. In this lattice, an atom in the center of the cell has all the eight corner atoms as its close neighbors. Hence the coordination number is eight.
Given below is the unit cell structure of ${\text{CsCl}}$. The ion in the center is the ${\text{C}}{{\text{s}}^ + }$ ion and it is surrounded by eight ${\text{C}}{{\text{l}}^ - }$ ions at the corners. Each ${\text{C}}{{\text{l}}^ - }$ ion is also surrounded by eight ${\text{C}}{{\text{s}}^ + }$ ions. Thus both have coordination numbers $8$.

Hence the correct option is C.
Note:
In bcc lattice, there are lattice points at eight corners and at the center of the unit cell. The atom at the center is independent of other cells, while each of eight atoms situated at the corners is shared by a total of eight unit cells. Thus the total number of atoms per unit cell$ = 1 + 8 \times \dfrac{1}{8} = 2$. So there are two atoms per unit cell of bcc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




