
In ${{C}}{{{O}}_{{2}}}$ molecule, ${{CO}}$ bond is polar but ${{C}}{{{O}}_{{2}}}$ molecule is nonpolar because the vector sum of dipole moment of the two ${{CO}}$ bond is zero.
A.True
B.False
Answer
565.2k+ views
Hint:The polarity of the bond is measured by its dipole moment. The molecule which has a dipole moment is called a polar molecule. The molecule which does not possess a dipole moment is called a nonpolar molecule. Molecules that have a polar covalent bond can be a polar or nonpolar molecule.
Complete step by step answer:
The dipole moment gives the measure of the polarity of the bond.
The dipole moment is a vector quantity.
It possesses both magnitude and direction.
Even if the molecule has polar bonds, if the dipole moment of the molecule is zero then the molecule is said to be non-polar.
If the molecule has a non-zero dipole moment then it is said to be a polar molecule.
When we consider the dipole moment of a molecule, we have to consider the vector sum of all dipoles in the molecule.
Whenever there is a separation of charge between two atoms in a bond, we can say that there exists a dipole.
Here in the question, it is said that the ${{CO}}$ bond in the \[{{C}}{{{O}}_{{2}}}\] molecule is polar. We know that oxygen in the ${{CO}}$ bond is more electronegative than carbon. So there is a separation of charge between carbon and oxygen in the ${{CO}}$ bond.
Thus there exists a dipole in the \[{{CO}}\] bond of the ${{C}}{{{O}}_{{2}}}$ molecule.
Since there exists a dipole in the ${{CO}}$ bond of the \[{{C}}{{{O}}_{{2}}}\], we can say that ${{CO}}$ bond is polar in the \[{{C}}{{{O}}_{{2}}}\] molecule.
As mentioned earlier dipole moment of the molecule is the vector sum of the individual dipoles in the molecule. That is the dipole moment depends both on magnitude and direction.
Therefore, for a molecule to be polar the vectors representing the dipole moment of each bond should not cancel out each other.
Here in the ${{C}}{{{O}}_{{2}}}$ molecule, there is no net dipole moment since the dipole moment vectors of the two ${{CO}}$ bond are in the opposite direction as shown below, it cancels out each other.
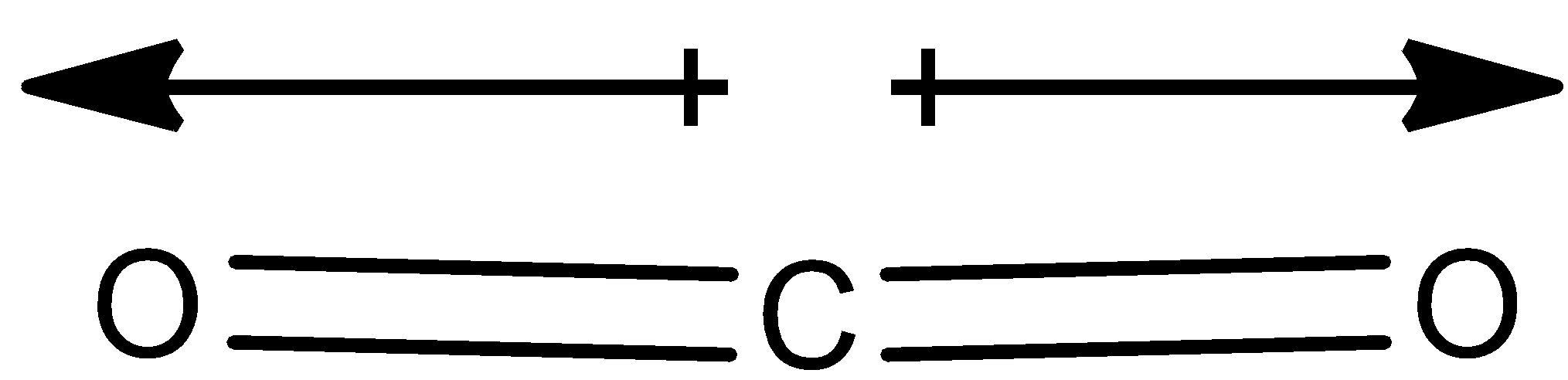
Therefore the net dipole moment of the ${{C}}{{{O}}_{{2}}}$ molecule is zero.
Thus ${{C}}{{{O}}_{{2}}}$ is non-polar.
The ${{CO}}$ bond in the ${{C}}{{{O}}_{{2}}}$ molecule is polar but the molecule ${{C}}{{{O}}_{{2}}}$ is nonpolar because the vector sum of the dipole moment of the two ${{CO}}$ bonds is zero.
Hence, the correct option is (A).
Note:
${{C}}{{{O}}_{{2}}}$ is a linear molecule. It is symmetric. Therefore the dipole moment of the two bonds cancel out each other and thus the net dipole moment is zero. Non-polar compounds are symmetric and polar molecules are asymmetric. Although the molecule contains a polar covalent bond, it is not a necessary condition for the molecule to be polar.
Complete step by step answer:
The dipole moment gives the measure of the polarity of the bond.
The dipole moment is a vector quantity.
It possesses both magnitude and direction.
Even if the molecule has polar bonds, if the dipole moment of the molecule is zero then the molecule is said to be non-polar.
If the molecule has a non-zero dipole moment then it is said to be a polar molecule.
When we consider the dipole moment of a molecule, we have to consider the vector sum of all dipoles in the molecule.
Whenever there is a separation of charge between two atoms in a bond, we can say that there exists a dipole.
Here in the question, it is said that the ${{CO}}$ bond in the \[{{C}}{{{O}}_{{2}}}\] molecule is polar. We know that oxygen in the ${{CO}}$ bond is more electronegative than carbon. So there is a separation of charge between carbon and oxygen in the ${{CO}}$ bond.
Thus there exists a dipole in the \[{{CO}}\] bond of the ${{C}}{{{O}}_{{2}}}$ molecule.
Since there exists a dipole in the ${{CO}}$ bond of the \[{{C}}{{{O}}_{{2}}}\], we can say that ${{CO}}$ bond is polar in the \[{{C}}{{{O}}_{{2}}}\] molecule.
As mentioned earlier dipole moment of the molecule is the vector sum of the individual dipoles in the molecule. That is the dipole moment depends both on magnitude and direction.
Therefore, for a molecule to be polar the vectors representing the dipole moment of each bond should not cancel out each other.
Here in the ${{C}}{{{O}}_{{2}}}$ molecule, there is no net dipole moment since the dipole moment vectors of the two ${{CO}}$ bond are in the opposite direction as shown below, it cancels out each other.
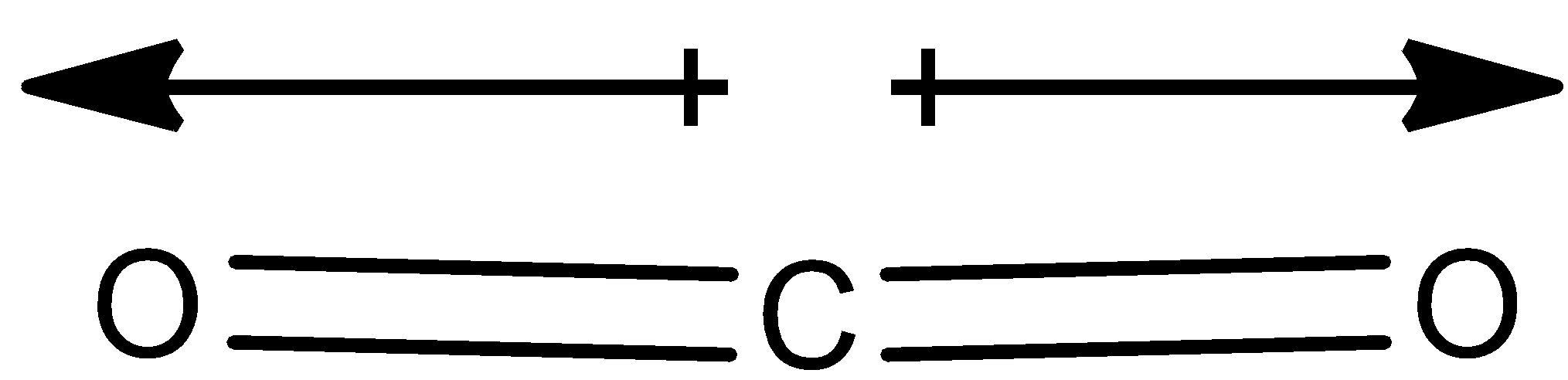
Therefore the net dipole moment of the ${{C}}{{{O}}_{{2}}}$ molecule is zero.
Thus ${{C}}{{{O}}_{{2}}}$ is non-polar.
The ${{CO}}$ bond in the ${{C}}{{{O}}_{{2}}}$ molecule is polar but the molecule ${{C}}{{{O}}_{{2}}}$ is nonpolar because the vector sum of the dipole moment of the two ${{CO}}$ bonds is zero.
Hence, the correct option is (A).
Note:
${{C}}{{{O}}_{{2}}}$ is a linear molecule. It is symmetric. Therefore the dipole moment of the two bonds cancel out each other and thus the net dipole moment is zero. Non-polar compounds are symmetric and polar molecules are asymmetric. Although the molecule contains a polar covalent bond, it is not a necessary condition for the molecule to be polar.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




