
In a reaction $\text{RCHO}$ is reduced to $\text{RC}{{\text{H}}_{3}}$ using amalgamated zinc and concentrated $\text{HCl}$ and warming the solution. The reaction is known as:
A. Meerwein-Ponndorf reaction
B. Clemmensen’s reduction
C. Wolff-kishner reduction
D. Schiff’s reaction
Answer
590.4k+ views
Hint: In this reaction, carbonyl group or $\left( \text{CO} \right)$ group is replaced by $\left( -\text{C}{{\text{H}}_{2}}- \right)$ group in the acidic medium. This reaction is helpful in obtaining alkyl chains directly from aldehydes and ketones. The answer can be obtained by knowing the general reactions of the options.
Complete step by step answer:
Let us discuss this question by looking at the options and writing its reactions:
A. Meerwein-Ponndorf reaction: In organic chemistry, this reaction is actually named as Meerwein-Ponndorf-Verley or MPV reduction is the reduction of ketones and aldehydes to alcohols using aluminium alkoxide catalysts in the presence of a sacrificial alcohol. The reaction is
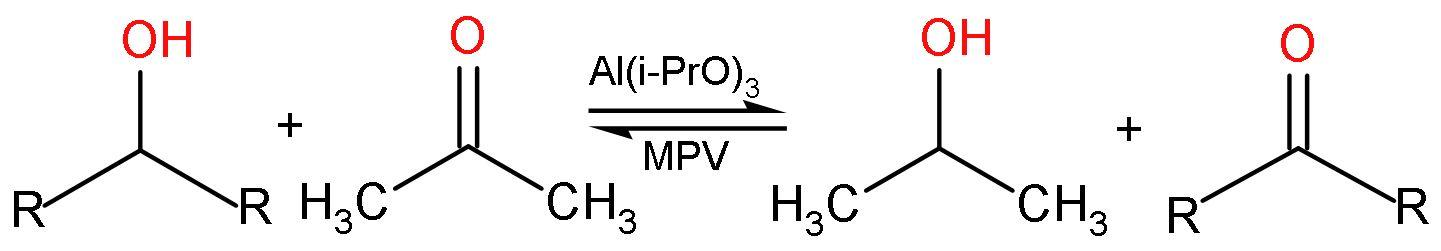
B. Clemmensen’s reduction: Clemmensen reduction is a chemical reaction in which aldehydes and ketones with zinc amalgam $\left( \text{Zn/Hg} \right)$ , which is an alloy of zinc and mercury in the acidic medium like concentrated hydrochloric acid $\left( \text{HCl} \right)$, which reduces the aldehydes and ketones to a hydrocarbon or $\left( -\text{C}{{\text{H}}_{2}}- \right)$ group. No new bond formation is taking place. The reaction is like
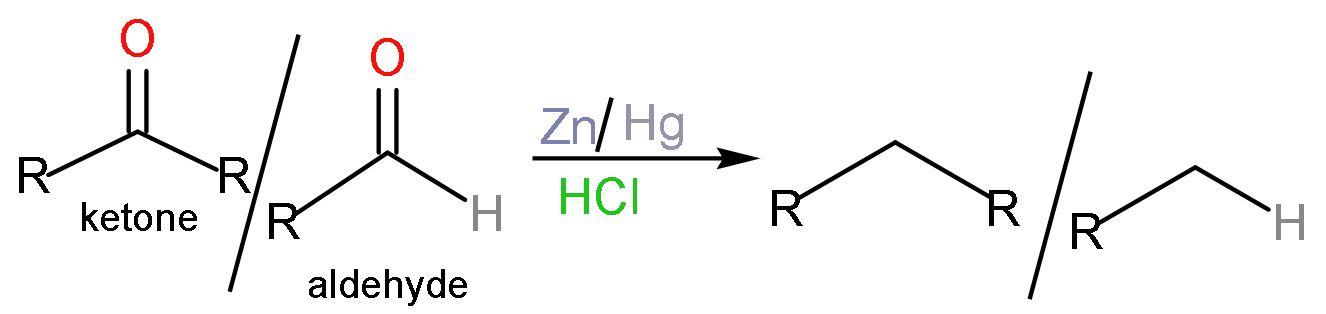
C. Wolff-kishner reduction: It is a reaction used to convert carbonyl group into methylene group. The reagents used in this reaction are hydrazine $\left( {{\text{H}}_{2}}\text{N}-\text{N}{{\text{H}}_{2}} \right)$ in the presence of base and heat, thus removing the nitrogen gas $\left( {{\text{N}}_{2}} \right)$. The reaction is

D. Schiff’s reaction: The Schiff test is a chemical test for detection of organic aldehydes that has also found use in the staining of biological tissues. The Schiff reagent is
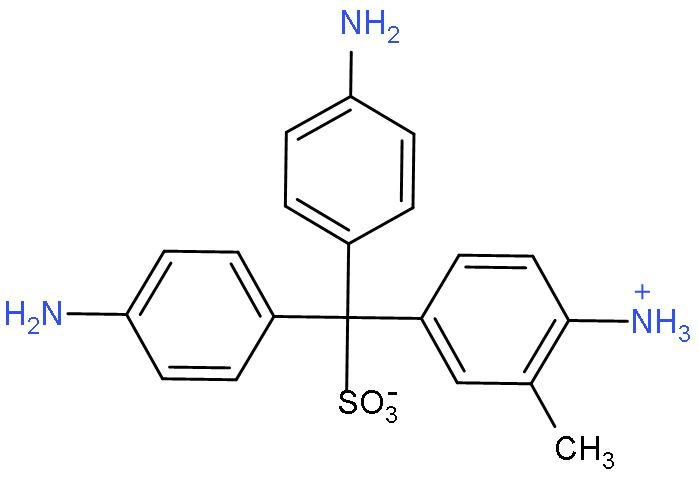
It is used as a qualitative test for aldehydes, the sample is added to the decolourized Schiff reagent. If aldehyde is present, the colour which appears is magenta colour.
In a reaction $\text{RCHO}$ is reduced to $\text{RC}{{\text{H}}_{3}}$ using amalgamated zinc and concentrated $\text{HCl}$ and warming the solution. The reaction is known as Clemmensen’s reduction. The option which matches is option ‘b’.
So, the correct answer is “Option B”.
Note: The Wolff-kishner reduction and Clemmensen’s reduction produce the same product but the difference lies in their reagents like Clemmensen’s reduction occurs in acidic medium whereas Wolff-kishner reduction occurs in basic medium.
If there is a compound in which aldehyde and hydroxyl groups are present and if you want to reduce the carbonyl group to $\left( -\text{C}{{\text{H}}_{2}}- \right)$. It can be done using Wolff-kishner reduction not Clemmensen’s reduction because in acidic medium, hydroxyl groups are removed to form alkenes.
Complete step by step answer:
Let us discuss this question by looking at the options and writing its reactions:
A. Meerwein-Ponndorf reaction: In organic chemistry, this reaction is actually named as Meerwein-Ponndorf-Verley or MPV reduction is the reduction of ketones and aldehydes to alcohols using aluminium alkoxide catalysts in the presence of a sacrificial alcohol. The reaction is

B. Clemmensen’s reduction: Clemmensen reduction is a chemical reaction in which aldehydes and ketones with zinc amalgam $\left( \text{Zn/Hg} \right)$ , which is an alloy of zinc and mercury in the acidic medium like concentrated hydrochloric acid $\left( \text{HCl} \right)$, which reduces the aldehydes and ketones to a hydrocarbon or $\left( -\text{C}{{\text{H}}_{2}}- \right)$ group. No new bond formation is taking place. The reaction is like

C. Wolff-kishner reduction: It is a reaction used to convert carbonyl group into methylene group. The reagents used in this reaction are hydrazine $\left( {{\text{H}}_{2}}\text{N}-\text{N}{{\text{H}}_{2}} \right)$ in the presence of base and heat, thus removing the nitrogen gas $\left( {{\text{N}}_{2}} \right)$. The reaction is

D. Schiff’s reaction: The Schiff test is a chemical test for detection of organic aldehydes that has also found use in the staining of biological tissues. The Schiff reagent is

It is used as a qualitative test for aldehydes, the sample is added to the decolourized Schiff reagent. If aldehyde is present, the colour which appears is magenta colour.
In a reaction $\text{RCHO}$ is reduced to $\text{RC}{{\text{H}}_{3}}$ using amalgamated zinc and concentrated $\text{HCl}$ and warming the solution. The reaction is known as Clemmensen’s reduction. The option which matches is option ‘b’.
So, the correct answer is “Option B”.
Note: The Wolff-kishner reduction and Clemmensen’s reduction produce the same product but the difference lies in their reagents like Clemmensen’s reduction occurs in acidic medium whereas Wolff-kishner reduction occurs in basic medium.
If there is a compound in which aldehyde and hydroxyl groups are present and if you want to reduce the carbonyl group to $\left( -\text{C}{{\text{H}}_{2}}- \right)$. It can be done using Wolff-kishner reduction not Clemmensen’s reduction because in acidic medium, hydroxyl groups are removed to form alkenes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




