
In $1909$, ____________ discovered nucleus in his famous gold foil experiment.
A. Rutherford
B. Goldstein
C. J.J. Thompson
D. None of the above
Answer
540k+ views
Hint: The person is a British physicist who hails from New Zealand and studied the atomic structure thereby giving the atomic model the name under which it was proposed in $1911$. The model is also known by the planetary models of the atom or nuclear atom.
Complete step by step answer:
After J J Thompson failed to explain the plum pudding model, a scientist named Ernest Rutherford performed an experiment by bombarding the $\alpha - $ particles to a thin sheet of gold foil from a radioactive source to study the deflection created by the $\alpha - $ particles.
The observations made by Rutherford were as follows;
Nucleus is the region where the positively charged particles are concentrated in the atom.
The model as shown in the diagram below depicts that the negatively charged electrons revolve around the nucleus in a circular manner, the circular path is called the orbit.
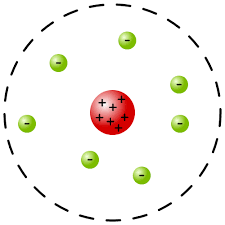
Therefore we can conclude that in 1909, Rutherford discovered nucleus in his famous gold foil experiment.
So, the correct answer is Option A.
Note: The atom’s stability was not clarified by Rutherfords’s atomic model which was a drawback of the model. The experiment was performed by fixing a fluorescent screen surrounding the gold foil where he found out that a large number of $\alpha - $ particles were passing through the gold foil without being deflected whereas some got deflected by small angles and very few deflected by ${180^0}$ angle.
Complete step by step answer:
After J J Thompson failed to explain the plum pudding model, a scientist named Ernest Rutherford performed an experiment by bombarding the $\alpha - $ particles to a thin sheet of gold foil from a radioactive source to study the deflection created by the $\alpha - $ particles.
The observations made by Rutherford were as follows;
Nucleus is the region where the positively charged particles are concentrated in the atom.
The model as shown in the diagram below depicts that the negatively charged electrons revolve around the nucleus in a circular manner, the circular path is called the orbit.

Therefore we can conclude that in 1909, Rutherford discovered nucleus in his famous gold foil experiment.
So, the correct answer is Option A.
Note: The atom’s stability was not clarified by Rutherfords’s atomic model which was a drawback of the model. The experiment was performed by fixing a fluorescent screen surrounding the gold foil where he found out that a large number of $\alpha - $ particles were passing through the gold foil without being deflected whereas some got deflected by small angles and very few deflected by ${180^0}$ angle.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




