
What is the hybridization state of boron atoms in the compound $ Mg\left[ {{B_2}O{{\left( {OH} \right)}_6}} \right] $ .
$ 1. $ Both $ s{p^3} $
$ 2. $ One $ s{p^2} $ and other $ s{p^3} $
$ 3. $ Both $ s{p^2} $
$ 4. $ One $ s{p^3} $ and other $ s{p^3}d $
Answer
502.8k+ views
Hint: There are two boron atoms present in the given compound. Therefore we will find hybridization of each boron atom. Hybridization is only valid for covalent compounds. So we will remove the ionic part first and then find hybridization of boron atoms.
Complete answer:
The given compound is a mixture of both ionic bonds and covalent bonds. Magnesium being a metal has two valence electrons in the outermost shell. Therefore it makes an ionic bond with the entity enclosed within the bracket. According to Molecular orbital theory, the orbitals overlap with other orbitals to form a hybrid orbital. Therefore, hybridization is only meant for compounds which are covalent in nature. Therefore we will first remove the ionic part from the given compound. Hence it can be represented as $ {\left[ {{B_2}O{{\left( {OH} \right)}_6}} \right]^{ - 2}} $ . Now this is purely covalent in nature. The hybridization of the compound can be depicted easily by looking at its structure. Hence the structure of the compound can be drawn as:
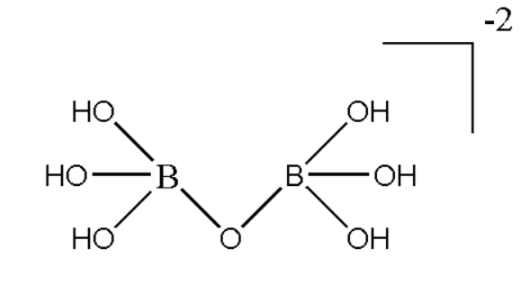
This is the structure of the given compound containing all covalent bonds formed by each boron atom. Here we can see that each boron atom forms three sigma bonds with $ OH $ and one sigma bond with $ O $ atom. Thus in total each boron atom makes four sigma bonds in the given structure. This means four orbitals are overlapping with orbitals of other atoms. Hence we can say that both boron are $ s{p^3} $ hybridized.
Note:
The hybridization of complex compounds is easy to depict by using the structure of that compound. Whenever there is any ionic part in the compound, firstly remove the ionic part because ionic bonds do not take part in hybridization of atoms.
Complete answer:
The given compound is a mixture of both ionic bonds and covalent bonds. Magnesium being a metal has two valence electrons in the outermost shell. Therefore it makes an ionic bond with the entity enclosed within the bracket. According to Molecular orbital theory, the orbitals overlap with other orbitals to form a hybrid orbital. Therefore, hybridization is only meant for compounds which are covalent in nature. Therefore we will first remove the ionic part from the given compound. Hence it can be represented as $ {\left[ {{B_2}O{{\left( {OH} \right)}_6}} \right]^{ - 2}} $ . Now this is purely covalent in nature. The hybridization of the compound can be depicted easily by looking at its structure. Hence the structure of the compound can be drawn as:

This is the structure of the given compound containing all covalent bonds formed by each boron atom. Here we can see that each boron atom forms three sigma bonds with $ OH $ and one sigma bond with $ O $ atom. Thus in total each boron atom makes four sigma bonds in the given structure. This means four orbitals are overlapping with orbitals of other atoms. Hence we can say that both boron are $ s{p^3} $ hybridized.
Note:
The hybridization of complex compounds is easy to depict by using the structure of that compound. Whenever there is any ionic part in the compound, firstly remove the ionic part because ionic bonds do not take part in hybridization of atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




