
What is the H - S - H bond angle in ${{H}_{2}}S$?
(A) \[{{104.5}^{0}}\]
(B) ${{92.1}^{0}}$
(C) ${{91}^{0}}$
(D) ${{90}^{0}}$
Answer
587.7k+ views
Hint: Draw the expanded structure of ${{H}_{2}}S$. Try to identify the number of lone pairs on the central atom i.e. sulphur in this case. Now find the hybridisation of the sulphur atom. The hybridisation will help to determine the shape of the compound and hence the bond angle of H - S - H.
Complete step by step solution:
Valence bond theory is one of the two theories along with the molecular orbital theory that was developed for the sole purpose of devising a mechanism to explain quantum mechanics of chemical bonding. According to the valence bond theory, a covalent bond is formed between two atoms by the overlap of half-filled valence atomic orbitals of the two atoms containing an unpaired electron each.
We will now draw the expanded structure of ${{H}_{2}}S$:
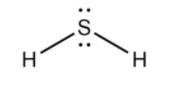
In the above structure, we observe that,
Number of bond pairs = 2
Number of lone pair of electrons = 2
The coordination number becomes 4. The hybridization is $\text{s}{{\text{p}}^{\text{3}}}$ and the shape of the molecule is bent.
Since the shape of the molecule is bent, the bond angle of H - S - H is ${{92.1}^{0}}$.
Therefore, the correct answer is option (B).
Note: Although the valence bond theory helps in predicting the shape and hybridisation of the molecule it has some drawbacks as well. Some of the limitations are mentioned below:
-Failure to explain the tetravalency exhibited by carbon
-No information regarding the energy possessed by electrons
-No clear distinction between weak and strong ligands
-No explanation regarding the colour of metal complexes
Complete step by step solution:
Valence bond theory is one of the two theories along with the molecular orbital theory that was developed for the sole purpose of devising a mechanism to explain quantum mechanics of chemical bonding. According to the valence bond theory, a covalent bond is formed between two atoms by the overlap of half-filled valence atomic orbitals of the two atoms containing an unpaired electron each.
We will now draw the expanded structure of ${{H}_{2}}S$:

In the above structure, we observe that,
Number of bond pairs = 2
Number of lone pair of electrons = 2
The coordination number becomes 4. The hybridization is $\text{s}{{\text{p}}^{\text{3}}}$ and the shape of the molecule is bent.
Since the shape of the molecule is bent, the bond angle of H - S - H is ${{92.1}^{0}}$.
Therefore, the correct answer is option (B).
Note: Although the valence bond theory helps in predicting the shape and hybridisation of the molecule it has some drawbacks as well. Some of the limitations are mentioned below:
-Failure to explain the tetravalency exhibited by carbon
-No information regarding the energy possessed by electrons
-No clear distinction between weak and strong ligands
-No explanation regarding the colour of metal complexes
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




