
Glyptal is the polymer of:
(A) Ethylene glycol
(B) Ethylene glycol and Phthalic acid
(C) Ethylene glycol and Adipic acid
(D) Caprolactam
Answer
597k+ views
Hint: Glyptal is a polyester type of polymer, meaning it contains ester functionality. It is prepared by step-growth polymerization. Glyptal is a co-polymer.
Complete Step-by-Step Answer:
If we know that Glyptal is a polyester type of polymer, then we can say that it involves ester as a functional group and so we can say that option (A) and (D) are not correct because polymerization of them will not result in ester functionality.
Ethylene glycol and Adipic acid polymerizes to give polymer Poly(ethylene adipate).
So, the correct answer is (B) Ethylene glycol and Phthalic acid.
Additional Information:
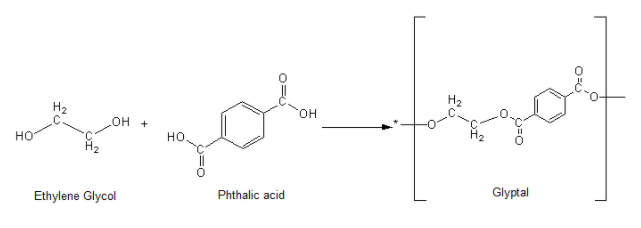
The structure of Glyptal is made up of two monomers Ethylene glycol and Phthalic acid as shown in the Reaction.
- It is a type of a copolymer as it is made by polymerization of two monomers.
- It is used in binding materials and cements.
- Ethylene glycol polymerizes to give Poly(ethylene glycol).
- Caprolactam is a cyclic amide and polymerizes to give Nylon-6 polymer.
Note: Other options given here also make other well known polymers but they do not make Glyptal. So, make sure that you do not make any errors. In case Glycerol is given as one of the options, make sure that you choose Ethylene glycol as one of the monomers because when both of them are present, mistakes can happen.
Complete Step-by-Step Answer:
If we know that Glyptal is a polyester type of polymer, then we can say that it involves ester as a functional group and so we can say that option (A) and (D) are not correct because polymerization of them will not result in ester functionality.
Ethylene glycol and Adipic acid polymerizes to give polymer Poly(ethylene adipate).
So, the correct answer is (B) Ethylene glycol and Phthalic acid.
Additional Information:

The structure of Glyptal is made up of two monomers Ethylene glycol and Phthalic acid as shown in the Reaction.
- It is a type of a copolymer as it is made by polymerization of two monomers.
- It is used in binding materials and cements.
- Ethylene glycol polymerizes to give Poly(ethylene glycol).
- Caprolactam is a cyclic amide and polymerizes to give Nylon-6 polymer.
Note: Other options given here also make other well known polymers but they do not make Glyptal. So, make sure that you do not make any errors. In case Glycerol is given as one of the options, make sure that you choose Ethylene glycol as one of the monomers because when both of them are present, mistakes can happen.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




